AMELUZ Gel Ref.[27465] Active ingredients: Aminolevulinic acid
Source: FDA, National Drug Code (US) Revision Year: 2021
Product description
AMELUZ (aminolevulinic acid hydrochloride) gel, 10% for topical use is a non-sterile white-to-yellowish gel. The gel formulation contains a nanoemulsion.
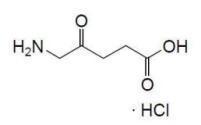
Aminolevulinic acid, a porphyrin precursor, is a white to off-white crystalline solid. It is readily soluble in water, methanol, and dimethylformamide. Its chemical name is 5-amino-4-oxo-pentanoic acid hydrochloride, molecular weight is 167.59 and molecular formula is C5H9NO3·HCl.
The structural formula of aminolevulinic acid hydrochloride is represented below:
Each gram of AMELUZ contains 100 mg of aminolevulinic acid hydrochloride (equivalent to 78 mg aminolevulinic acid) as the active ingredient and the following inactive ingredients: xanthan gum, soybean phosphatidylcholine, polysorbate 80, medium-chain triglycerides, isopropyl alcohol, dibasic sodium phosphate, monobasic sodium phosphate, propylene glycol, sodium benzoate and purified water.
| Dosage Forms and Strengths |
|---|
|
Each gram of AMELUZ gel, 10% contains 100 mg of aminolevulinic acid hydrochloride (equivalent to 78 mg of aminolevulinic acid). |
| How Supplied |
|---|
|
AMELUZ (aminolevulinic acid hydrochloride) gel, 10% is a white-to-yellowish gel. The drug product is supplied in an aluminum tube with a white, high density polyethylene (HDPE) screw cap. Each tube contains 2 g of gel. NDC 70621-101-01 2 g tube Manufacturer: Biofrontera Pharma GmbH Distributed by: Biofrontera Inc., 120 Presidential Way, Suite 330, Woburn, MA 01801, USA |
Drugs
| Drug | Countries | |
|---|---|---|
| AMELUZ | Austria, Estonia, Spain, Croatia, Ireland, Lithuania, Poland, United Kingdom, United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.