ANZATAX Concentrate for solution for injection Ref.[50346] Active ingredients: Paclitaxel
Source: Pharmaceutical Benefits Scheme (AU) Revision Year: 2021 Publisher: Pfizer Australia Pty Ltd, Level 17, 151 Clarence Street, Sydney NSW 2000, Toll Free number: 1800 675 229, www.pfizer.com.au
Product name and form
ANZATAXT – Paclitaxel.
| Pharmaceutical Form |
|---|
|
Concentrate for solution for injection. Anzatax Injection Concentrate has a pH of 6 to 7. It is a clear to pale yellow solution, free of visible particles. |
Qualitative and quantitative composition
Paclitaxel is an anticancer agent from the taxane class of drugs.
Anzatax Injection Concentrate is a sterile solution containing 6 mg/mL paclitaxel. It is a white powder. Paclitaxel is extremely hydrophobic, and is therefore formulated in PEG-35 castor oil and ethanol.
Excipients with known effect: Ethanol
For the full list of excipients, see section 6.1 List of excipients.
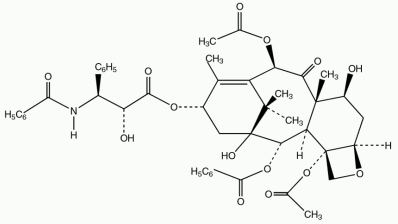
Chemical structure
Paclitaxel is described chemically as (2S,5R,7S,10R,13S)-10,20-bis(acetoxy)-2-benzoyloxy1,7-dihydroxy-9-oxo-5,20-epoxytax-11-en-13-yl (3S)-3-benzoylamino-3-phenyl-D-lactate.
The chemical structure of Paclitaxel is shown below:
Molecular weight: 853.9
CAS number: 33069-62-4
| Active Ingredient |
|---|
|
Paclitaxel is an antimicrotubule agent that promotes the assembly of microtubules from tubulin dimers and stabilises microtubules by preventing depolymerisation. This stability results in the inhibition of the normal dynamic reorganisation of the microtubule network that is essential for vital interphase and mitotic cellular functions. |
| List of Excipients |
|---|
|
Citric acid |
Pack sizes and marketing
Anzatax Injection Concentrate is available in glass vial in single packs in the following mpresentations:
Anzatax Injection Concentrate 30 mg/5 mL vials.
Anzatax Injection Concentrate 100 mg/16.7 mL vials.
Anzatax Injection Concentrate 150 mg/25 mL vials.
Anzatax Injection Concentrate 300 mg/50 mL vials.
Marketing authorization holder
Pfizer Australia Pty Ltd, Level 17, 151 Clarence Street, Sydney NSW 2000, Toll Free number: 1800 675 229, www.pfizer.com.au
Marketing authorization dates and numbers
30 mg/5 mL: 12 January 1995
100 mg/16.7 mL: 24 February 2005
150 mg/25 mL: 12 January 1995
300 mg/50 mL: 7 January 2003
Drugs
| Drug | Countries | |
|---|---|---|
| ANZATAX | Hong Kong, New Zealand, Singapore, Tunisia, Turkey, South Africa |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.