APEALEA Powder for solution for infusion Ref.[49913] Active ingredients: Paclitaxel
Source: European Medicines Agency (EU) Revision Year: 2022 Publisher: Inceptua AB, Gustavslundsv. 143, 16751 Bromma, Sweden
5.1. Pharmacodynamic properties
Pharmacotherapeutic group: Antineoplastic agents, taxanes
ATC code: L01CD01
Mechanism of action
Paclitaxel is an antimicrotubule agent that promotes the assembly of microtubules from tubulin dimers and stabilises microtubules by preventing depolymerisation. Stabilisation results in the inhibition of the normal dynamic reorganisation of the microtubule network that is essential for vital interphase and mitotic cellular functions. In addition, paclitaxel induces microtubule bundle formation throughout the cell cycle and induces microtubule aster formation during mitosis.
Clinical efficacy and safety
An open, randomised, multicentre study was conducted in 789 women with recurrent epithelial ovarian cancer, primary peritoneal cancer and fallopian tube cancer to compare Apealea (paclitaxel micellar) in combination with carboplatin with solvent-based paclitaxel in combination with carboplatin. Patients were treated every three weeks for six cycles, either with Apealea 250 mg/m² given as a 1-hour intravenous infusion (N=391) or with solvent-based paclitaxel 175 mg/m² given as a 3-hour infusion (N=391). The paclitaxel infusion was followed by carboplatin after an interval of 30 minutes in both treatment arms.
Patients were stratified based on relapse (first or second) and CA125 values. The proportions of patients treated at first or second relapse were then equal in both treatment groups (76% were treated at first relapse and 24% at second relapse). Patients who had pre-existing neuropathy of grade ≥2 or serious medical risk factors involving any of the major organ systems were not allowed to enter the study. The mean age was 56 years of age in both treatment groups (range 26–81). Most of the patients enrolled in the study had ECOG performance status of 0 or 1 (≥96%), in similar proportions between the treatment arms. Only a few patients had ECOG performance status of 2.
In the clinical study, the proportion of patients receiving six treatment cycles was 81% in the group treated with paclitaxel micellar and 87% in the group receiving solvent-based paclitaxel. The corresponding median number of cycles (min;max) for the two groups were 6 (1;12) and 6 (1;9), respectively.
Patients received premedication prior to infusion of solvent-based paclitaxel, paclitaxel micellar and carboplatin as summarised in Table 3 below. Premedication was not mandated prior to infusion of paclitaxel micellar.
Table 3. Proportions of patients who received premedication prior to infusion of paclitaxel or carboplatin or overall (safety population):
| Apealea (N=391) | Paclitaxel (solvent-based) (N=391) | |||||
|---|---|---|---|---|---|---|
| Type of premedication | Overall | Paclitaxel | Carboplatin | Overall | Paclitaxel | Carboplatin |
| Corticosteroids | 43% | 6% | 39% | 99% | 97% | 15% |
| Antihistamines | 19% | 4% | 16% | 85% | 85% | 9% |
| H2 antagonists | 5% | 2% | 2% | 90% | 90% | 1% |
| Antiemetics and antinauseants | 87% | 8% | 81% | 92% | 38% | 63% |
In the study, 35% of the patients in the paclitaxel micellar group and 30% of the patients in the solvent-based paclitaxel group, respectively, received GCSF to treat neutropenia. The median number of cycles with paclitaxel/carboplatin treatment for patients receiving GCSF was 6 in both groups. The median number of cycles with administration of GCSF was 3 (1;6) and the mean value 3.1, each in both groups.
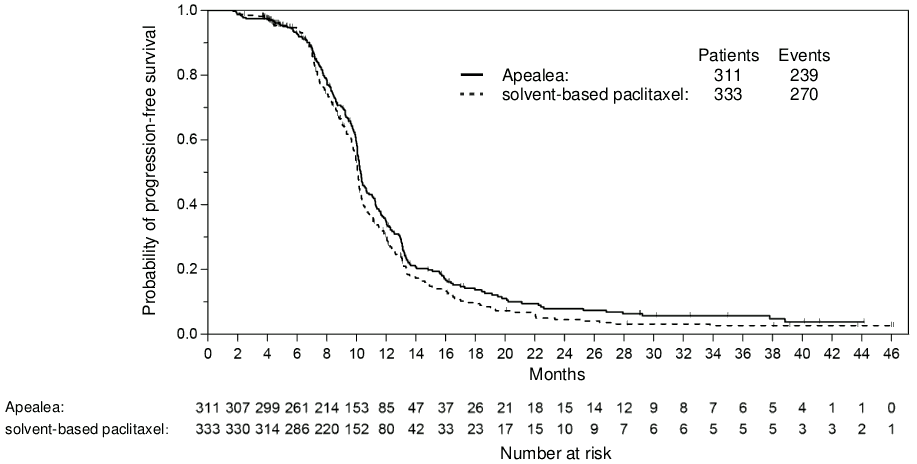
The principle measures of efficacy were progression-free survival (PFS) and overall survival (OS). PFS as the primary endpoint was evaluated by blinded assessment of computerised tomography images using Response Evaluation Criteria in Solid Tumours (RECIST) 1.0.
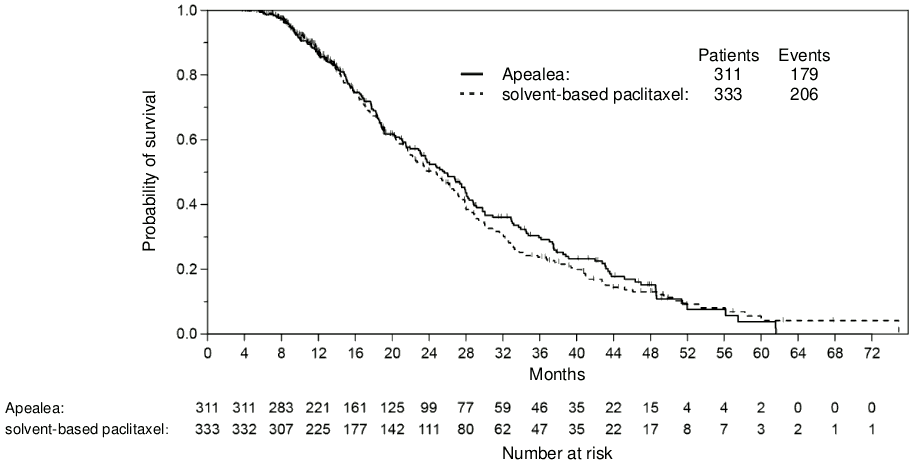
There was no statistically significant difference in PFS or OS between the two treatment arms. A non-inferiority analysis was conducted for PFS in the per-protocol (PP) population with pre-specified non-inferiority margin. The non-inferiority criterion was met for PFS with the upper bound limit of the one-sided 97.5% confidence interval (CI) for the associated hazard ratio being less than 1.2. The non-inferiority criterion was met for OS in the PP population with the upper bound limit of the one-sided 97.5% CI for the associated hazard ratio being less than 1.185 (Table 4; Figures 1 and 2). In the intention-to-treat (ITT) population (n=789), the hazard ratios for PFS and OS were 0.85 (95% CI: 0.72;1.00) and 1.02 (95% CI: 0.85;1.22), respectively. Thereby, non-inferiority was shown in the ITT population for PFS, but not for OS. At the time of analysis of the OS data, death had occurred in 56% of the patients in the group treated with paclitaxel micellar compared to 60% in the group treated with solvent-based paclitaxel (ITT population).
Table 4. Non-inferiority analyses on PFS and OS in a randomised trial in patients with recurrent epithelial ovarian cancer, primary peritoneal cancer and fallopian tube cancer (PP population)a:
| Apealea Q3W 250 mg/m² + carboplatin (N=311) | Solvent-based paclitaxel Q3W 175 mg/m² + carboplatin (N=333) | |
|---|---|---|
| Progression free survival (independent review) | ||
| Death or progression, n (%) | 239 (77%) | 270 (81%) |
| Median time to death or disease progression [months] (95% CI) | 10.3 (10.1;10.7) | 10.1 (9.9;10.2) |
| Hazard ratio (95% CI) | 0.86 (0.72;1.03) | |
| Overall survival | ||
| Number of deaths, n (%) | 179 (58%) | 206 (62%) |
| Median time to death [months] (95% CI) | 25.7 (22.9;28.1) | 24.8 (21.7;27.1) |
| Hazard ratio (95% CI) | 0.95 (0.78;1.16) | |
a Primary population in non-inferiority analysis was predefined as the PP population.
Figure 1. Kaplan-Meier curve of PFS (PP population):
Figure 2. Kaplan-Meier curve of OS (PP population):
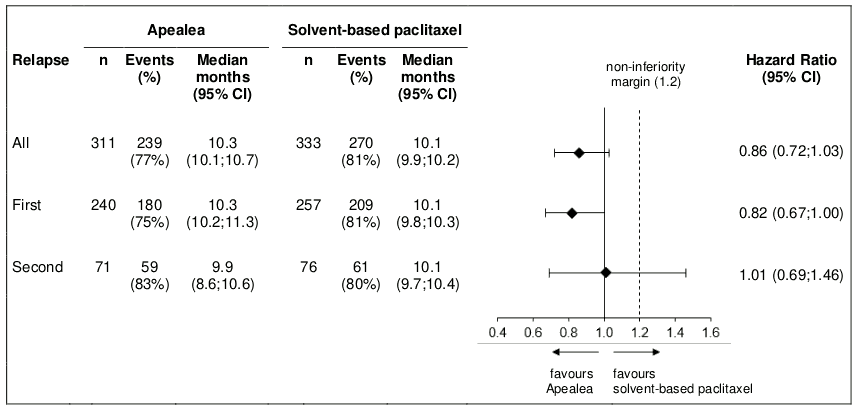
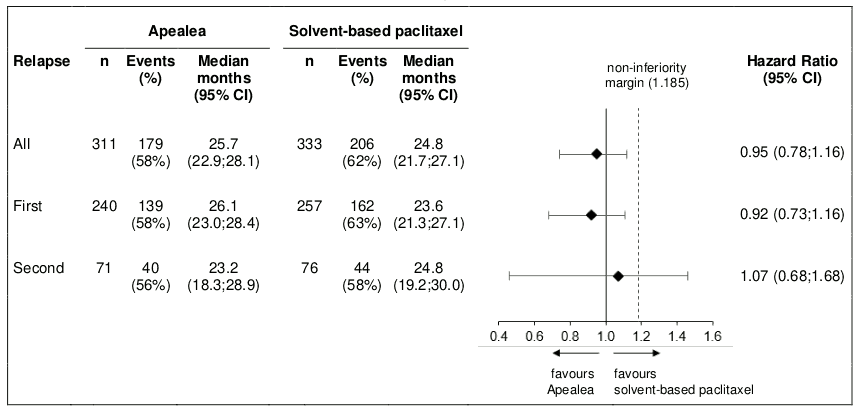
Post-hoc subgroup analysis by relapse
Additional subgroup analyses were conducted to investigate efficacy by relapse (first and second) in the PP and the ITT population. PFS and OS results in the PP population are summarised in Figures 3 and 4.
Figure 3. Forest plot for PFS by relapse (PP population):
Figure 4. Forest plot for OS by relapse (PP population):
In the ITT population, the hazard ratios for PFS in the subgroups of patients with first relapse and second relapse were 0.80 (95% CI: 0.66;0.97) and 1.04 (95% CI: 0.74;1.47), respectively. The hazard ratios for OS in patients with first and second relapse were 0.98 (95% CI: 0.79;1.21) and 1.18 (95% CI: 0.79;1.75), respectively. Thus, the results in the subgroup of patients with first relapse are consistent with the results in the overall population and in addition, there was an indication of PFS benefit for Apealea.
For safety data comparing the results of combination treatment with Apealea (paclitaxel micellar)/carboplatin and solvent-based paclitaxel/carboplatin, see section 4.8.
Paediatric population
The European Medicines Agency has waived the obligation to submit the results of studies with Apealea in all subsets of the paediatric population in the treatment of ovarian carcinoma (excluding rhabdomyosarcoma and germ cell tumours), peritoneal carcinoma (excluding blastomas and sarcomas) and fallopian tube carcinoma (excluding rhabdomyosarcoma and germ cell tumours) (see section 4.2 for information on paediatric use).
5.2. Pharmacokinetic properties
When Apealea (paclitaxel micellar) is administered intravenously, its pharmacokinetic profile suggests that the formulation immediately releases paclitaxel into the blood. The pharmacokinetics of paclitaxel were studied in 22 patients with solid tumours after 1-hour infusions of Apealea (dose levels of 90 to 275 mg/m²). In addition, a study with a crossover design compared total and unbound paclitaxel concentrations in plasma after a 1-hour infusion of Apealea 260 mg/m² with those after a 1-hour infusion of albumin-bound paclitaxel at the same dose. Total plasma levels of paclitaxel were similar after infusion of the two formulations. The plasma levels of unbound paclitaxel, i.e. the concentration that represents pharmacologically active paclitaxel in the body, were demonstrated to be bioequivalent (Cmax and AUC) after administration of albumin-bound paclitaxel and Apealea. Based on limited data, Cmax and AUC increased with dose after 1-hour infusions of Apealea in doses ranging from 150 to 275 mg/m². Dose-linearity could not be ascertained since a large inter-individual variability was observed.
Distribution
Paclitaxel is distributed equally between plasma and blood as described in published in vitro data. The mean unbound fraction of paclitaxel (fu) varied between 5.2% and 4.3% over time after Apealea infusion. This was in agreement with the mean fu after albumin-bound paclitaxel infusion which varied between 5.5% and 4.5% over time.
Binding of paclitaxel to both albumin and α1-acid glycoprotein has been reported, but other binding proteins such as lipoproteins might be important. There are no reports of active substances able to displace protein-bound paclitaxel, nor is paclitaxel a likely candidate as a displacer of other active substances given its low molar concentrations in plasma. Based on the published literature, in vitro studies indicate that the presence of cimetidine, ranitidine, dexamethasone, or diphenhydramine does not affect protein binding of paclitaxel. Paclitaxel has been shown in vitro to be a substrate for the influx transporter proteins OATP1B3 and OATP1A2.
During and after infusion of Apealea, paclitaxel rapidly leaves the plasma compartment with a distribution half-life of about 0.6 hours. Thus, the distribution phase is essentially complete at 2 hours after the end of infusion. The tissue distribution is extensive, with a volume of distribution on the terminal elimination phase of about 155 L/m² corresponding to about 280 L for an average patient with a body surface area of 1.8 m². Thus, only about 1% of the paclitaxel in the body is located in plasma during the elimination phase.
Biotransformation and elimination
The terminal half-life of paclitaxel after Apealea infusion varied about 5-fold between the subjects, 5–23 hours. Likewise, total plasma clearance varied about 5-fold from 8 to 41 L/hour. The high interindividual variability in clearance is believed to be a consequence of variability in hepatic enzyme activity.
The biotransformation and elimination of paclitaxel have been reported in published studies; paclitaxel is mainly eliminated by hepatic metabolism and biliary excretion. The main metabolite of paclitaxel is 6α-hydroxypaclitaxel. Other metabolites are 3'-p-hydroxypaclitaxel and 6α,3'-p-dihydroxypaclitaxel. The formation of these metabolites is catalysed by CYP2C8 and CYP3A4. No pharmacologically active metabolite has been found. In vitro and in vivo studies have demonstrated that paclitaxel is a substrate for the efflux protein P-glycoprotein. The major route of excretion of paclitaxel-derived material in humans is the faeces, where 6α-hydroxypaclitaxel constitutes the main material. Renal excretion accounts for a minor part, less than 15% of the dose.
Special populations
Hepatic impairment
No clinical studies in patients with hepatic impairment have been undertaken with Apealea (see sections 4.2 and 4.4). A population pharmacokinetics study with albumin-bound paclitaxel demonstrated that patients with mild hepatic impairment (total bilirubin >1 to ≤1.5 × ULN) have an elimination rate in the normal range. In contrast, patients with moderate hepatic impairment (total bilirubin >1.5 to ≤3 × ULN) and severe hepatic impairment (total bilirubin >3 to ≤5 × ULN) had a 22% or a 26% reduction in paclitaxel elimination rate, respectively. Compared to patients with normal hepatic function, hepatically impaired patients with total bilirubin >1.5 × ULN have an increase in mean paclitaxel AUC of approximately 20%. Hepatic impairment has no effect on mean paclitaxel Cmax. Pharmacokinetic data for patients with total bilirubin >5 × ULN are not available.
Renal impairment
No clinical studies in patients with renal impairment have been undertaken with Apealea (see section 4.2 for dose recommendations). Since renal elimination is a minor pathway for paclitaxel, increased plasma levels are not expected in this patient group. A population pharmacokinetics study with albumin-bound paclitaxel demonstrated that patients with mild and moderate renal impairment (creatinine clearance ≥30 to <90 mL/min) have an elimination rate similar to that of patients with normal renal function. Information is lacking for patients with severe renal impairment (GFR <30 mL/min).
Effects of age, gender, race and body size
No analysis of the effect of age, gender or body size on the elimination of Apealea has been undertaken. However, a population pharmacokinetics study of 168 patients (86 males and 82 females) treated with solvent-based paclitaxel has been reported. On average, the paclitaxel elimination rate was 20% higher in males compared to in females. With regard to age, the population model indicated an approximate 5% decline in paclitaxel elimination rate for each 10-year increase in age compared to the median age of 56 years of the study. This amounted to a 14% decline in an 86-year-old patient compared to one aged 56. Further it has been shown that the rate of paclitaxel elimination increased with increasing body size. The model indicated that a 0.2 m² increase in BSA would lead to a 9% increase in the elimination rate. There is very little information available on whether the elimination of paclitaxel differs between races.
5.3. Preclinical safety data
Mutagenesis, carcinogenesis, impairment of fertility
In vitro studies using different cell systems have shown paclitaxel to be clastogenic inducing chromosomal aberrations, micronuclei and DNA damage. Chromosomal aberrations have also been detected in in vivo studies in mice and monkeys. Paclitaxel was devoid of mutagenic activity in the Ames test or the Chinese hamster ovary/hypoxanthine-guanine phosphoribosyl transferase (CHO/HGPRT) gene mutation assay. The carcinogenic activity of paclitaxel has not been studied. However, paclitaxel is potentially carcinogenic based on its mechanism of action and demonstrated genotoxic activity. Paclitaxel at doses below the human therapeutic dose was associated with low fertility and foetal toxicity in rats. Repeat dose toxicity studies have shown non-reversible, toxic effects on male reproductive organs.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.