ATACAND HCT Tablet Ref.[50626] Active ingredients: Candesartan Hydrochlorothiazide
Source: FDA, National Drug Code (US) Revision Year: 2020
Product description
ATACAND HCT (candesartan cilexetil-hydrochlorothiazide) combines an angiotensin II receptor (type AT1) antagonist and a diuretic, hydrochlorothiazide.
Candesartan cilexetil, a nonpeptide, is chemically described as (±)-1-Hydroxyethyl 2-ethoxy-1-[p-(o-1H-tetrazol-5-ylphenyl)benzyl]-7-benzimidazolecarboxylate, cyclohexyl carbonate (ester).
Its empirical formula is C33H34N6O6 and its structural formula is:
Candesartan cilexetil is a white to off-white powder with a molecular weight of 610.67. It is practically insoluble in water and sparingly soluble in methanol. Candesartan cilexetil is a racemic mixture containing one chiral center at the cyclohexyloxycarbonyloxy ethyl ester group. Following oral administration, candesartan cilexetil undergoes hydrolysis at the ester link to form the active drug, candesartan, which is achiral.
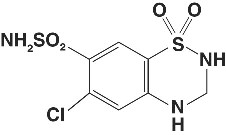
Hydrochlorothiazide is 6-chloro-3,4-dihydro-2H-1,2,4-benzothiadiazine-7-sulfonamide 1,1-dioxide. Its empirical formula is C7H8ClN3O4S2 and its structural formula is:
Hydrochlorothiazide is a white, or practically white, crystalline powder with a molecular weight of 297.72, which is slightly soluble in water, but freely soluble in sodium hydroxide solution.
ATACAND HCT is available for oral administration in three tablet strengths of candesartan cilexetil and hydrochlorothiazide.
ATACAND HCT 16-12.5 contains 16 mg of candesartan cilexetil and 12.5 mg of hydrochlorothiazide. ATACAND HCT 32-12.5 contains 32 mg of candesartan cilexetil and 12.5 mg of hydrochlorothiazide. ATACAND HCT 32-25 contains 32 mg of candesartan cilexetil and 25 mg of hydrochlorothiazide. The inactive ingredients of the tablets are carboxymethylcellulose calcium, hydroxypropyl cellulose, lactose monohydrate, magnesium stearate, cornstarch, polyethylene glycol 8000, and ferric oxide (yellow). Ferric oxide (reddish brown) is also added to the 16-12.5 mg and 32-25 mg tablets as colorant.
| How Supplied |
|---|
|
ATACAND HCT 16-12.5 mg: Tablets are peach, oval, biconvex, non-film-coated tablets, scored on both sides and coded with ACS on one side. They are supplied in bottles of 90 tablets (NDC 62559-650-90). ATACAND HCT 32-12.5 mg: Tablets are yellow, oval, biconvex, non-film-coated tablets, scored on both sides and coded with ACJ on one side. They are supplied in bottles of 90 tablets (NDC 62559-651-90). ATACAND HCT 32-25 mg: Tablets are pink, oval, biconvex, non-film-coated tablets, scored on both sides and coded with ACD on one side. They are supplied in bottles of 90 tablets (NDC 62559-652-90). |
Drugs
| Drug | Countries | |
|---|---|---|
| ATACAND HCT | Brazil, United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.