ATOZET Film-coated tablet Ref.[50752] Active ingredients: Atorvastatin Atorvastatin and Ezetimibe Ezetimibe
Source: Health Products Regulatory Authority (IE) Revision Year: 2023 Publisher: N.V. Organon, Kloosterstraat 6, NL-5349 AB Oss, The Netherlands
5.1. Pharmacodynamic properties
Pharmacotherapeutic group: Lipid modifying agents, HMG-CoA reductase inhibitors in combination with other lipid modifying agents
ATC code: C10BA05
ATOZET (ezetimibe/atorvastatin) is a lipid-lowering product that selectively inhibits the intestinal absorption of cholesterol and related plant sterols and inhibits the endogenous synthesis of cholesterol.
Mechanism of action
ATOZET
Plasma cholesterol is derived from intestinal absorption and endogenous synthesis. ATOZET contains ezetimibe and atorvastatin, two lipid-lowering compounds with complementary mechanisms of action. ATOZET reduces elevated total cholesterol (total-C), LDL-C, apolipoprotein B (Apo B), triglycerides (TG), and non-high-density lipoprotein cholesterol (non-HDL-C), and increases high-density lipoprotein cholesterol (HDL-C) through dual inhibition of cholesterol absorption and synthesis.
Ezetimibe
Ezetimibe inhibits the intestinal absorption of cholesterol. Ezetimibe is orally active and has a mechanism of action that differs from other classes of cholesterol-reducing compounds (e.g. statins, bile acid sequestrants [resins], fibric acid derivatives, and plant stanols). The molecular target of ezetimibe is the sterol transporter, Niemann-Pick C1-Like 1 (NPC1L1), which is responsible for the intestinal uptake of cholesterol and phytosterols.
Ezetimibe localises at the brush border of the small intestine and inhibits the absorption of cholesterol, leading to a decrease in the delivery of intestinal cholesterol to the liver; statins reduce cholesterol synthesis in the liver and together these distinct mechanisms provide complementary cholesterol reduction. In a 2-week clinical study in 18 hypercholesterolaemic patients, ezetimibe inhibited intestinal cholesterol absorption by 54%, compared with placebo.
A series of preclinical studies was performed to determine the selectivity of ezetimibe for inhibiting cholesterol absorption. Ezetimibe inhibited the absorption of [14C]-cholesterol with no effect on the absorption of triglycerides, fatty acids, bile acids, progesterone, ethinyl estradiol, or fat soluble vitamins A and D.
Atorvastatin
Atorvastatin is a selective, competitive inhibitor of HMG-CoA reductase, the rate-limiting enzyme responsible for the conversion of 3-hydroxy-3-methyl-glutaryl-coenzyme A to mevalonate, a precursor of sterols, including cholesterol. Triglycerides and cholesterol in the liver are incorporated into very low-density lipoproteins (VLDL) and released into the plasma for delivery to peripheral tissues. Low-density lipoprotein (LDL) is formed from VLDL and is catabolised primarily through the receptor with high affinity to LDL (LDL receptor).
Atorvastatin lowers plasma cholesterol and lipoprotein serum concentrations by inhibiting HMG-CoA reductase and subsequently cholesterol biosynthesis in the liver and increases the number of hepatic LDL receptors on the cell surface for enhanced uptake and catabolism of LDL.
Atorvastatin reduces LDL production and the number of LDL particles. Atorvastatin produces a profound and sustained increase in LDL receptor activity coupled with a beneficial change in the quality of circulating LDL particles. Atorvastatin is effective in reducing LDL-C in patients with homozygous familial hypercholesterolaemia, a population that has not usually responded to lipid-lowering medicinal products.
Atorvastatin has been shown to reduce concentrations of total-C (30% - 46%), LDL-C (41% - 61%), apolipoprotein B (34% - 50%), and triglycerides (14% - 33%) while producing variable increases in HDL-C and apolipoprotein A1 in a dose response study. These results are consistent in patients with heterozygous familial hypercholesterolaemia, nonfamilial forms of hypercholesterolaemia, and mixed hyperlipidaemia, including patients with noninsulin-dependent diabetes mellitus.
Clinical efficacy and safety
In controlled clinical studies, ATOZET significantly reduced total-C, LDL-C, Apo B, and TG, and increased HDL-C in patients with hypercholesterolaemia.
Primary Hypercholesterolaemia
In a placebo-controlled study, 628 patients with hyperlipidaemia were randomised to receive placebo, ezetimibe (10 mg), atorvastatin (10 mg, 20 mg, 40 mg, or 80 mg), or co-administered ezetimibe and atorvastatin equivalent to ATOZET (10/10, 10/20, 10/40, and 10/80) for up to 12-weeks.
Patients receiving all doses of ATOZET were compared to those receiving all doses of atorvastatin. ATOZET lowered total-C, LDL-C, Apo B, TG, and non-HDL-C, and increased HDL-C significantly more than atorvastatin alone. (See Table 4.)
Table 4. Response to ATOZET in Patients with Primary Hyperlipidaemia (Meana % Change from Untreated Baselineb at 12 weeks):
| Treatment (Daily Dose) | N | Total-C | LDL-C | Apo B | TGa | HDL-C | Non-HDL-C |
|---|---|---|---|---|---|---|---|
| Pooled data (All ATOZET doses)c | 255 | -41 | -56 | -45 | -33 | +7 | -52 |
| Pooled data (All atorvastatin doses)c | 248 | -32 | -44 | -36 | -24 | +4 | -41 |
| Ezetimibe 10 mg | 65 | -14 | -20 | -15 | -5 | +4 | -18 |
| Placebo | 60 | +4 | +4 | +3 | -6 | +4 | +4 |
| ATOZET by dose | |||||||
| 10/10 | 65 | -38 | -53 | -43 | -31 | +9 | -49 |
| 10/20 | 62 | -39 | -54 | -44 | -30 | +9 | -50 |
| 10/40 | 65 | -42 | -56 | -45 | -34 | +5 | -52 |
| 10/80 | 63 | -46 | -61 | -50 | -40 | +7 | -58 |
| Atorvastatin by dose | |||||||
| 10 mg | 60 | -26 | -37 | -28 | -21 | +6 | -34 |
| 20 mg | 60 | -30 | -42 | -34 | -23 | +4 | -39 |
| 40 mg | 66 | -32 | -45 | -37 | -24 | +4 | -41 |
| 80 mg | 62 | -40 | -54 | -46 | -31 | +3 | -51 |
a For triglycerides, median % change from baseline
b Baseline – on no lipid-lowering drug
c ATOZET pooled (10/10-10/80) significantly reduced total-C, LDL-C, Apo B, TG, non-HDL-C, and significantly increased HDL-C compared to all doses of atorvastatin pooled (10-80 mg).
In a controlled study, the Titration of Atorvastatin vs Ezetimibe Add-On to Atorvastatin in Patients with Hypercholesterolaemia (TEMPO) study, 184 patients, with an LDL-C level ≥2.6 mmol/L and ≥4.1 mmol/L and at moderate high risk for CHD, received atorvastatin 20 mg for a minimum of 4 weeks prior to randomisation. Patients not at an LDL-C level <2.6 mmol/L were randomised to receive either co-administered ezetimibe and atorvastatin (equivalent to ATOZET 10/20) or atorvastatin 40 mg for 6 weeks.
ATOZET 10/20 was significantly more effective than doubling the dose of atorvastatin to 40 mg in further reducing total-C (-20% vs. -7%), LDL-C (-31% vs. -11%), Apo B (-21% vs. -8%), and nonHDL-C (-27% vs. -10%). Results for HDL-C and TG between the two treatment groups were not significantly different. Also, significantly more patients receiving ATOZET 10/20 attained LDL-C <2.6 mmol/L compared to those receiving atorvastatin 40 mg, 84% vs. 49%.
In a controlled study, The Ezetimibe Plus Atorvastatin vs Atorvastatin Titration in Achieving Lower LDL-C Targets in Hypercholesterolaemic Patients (EZ-PATH) study, 556 high-cardiovascular-risk patients with a LDL-C level ≥1.8 mmol/L and ≤4.1 mmol/L received atorvastatin 40 mg for a minimum of 4 weeks prior to randomisation. Patients not at a LDL-C level <1.8 mmol/L were randomised to receive either co-administered ezetimibe and atorvastatin (equivalent to ATOZET 10/40) or atorvastatin 80 mg for 6 weeks.
ATOZET 10/40 was significantly more effective than doubling the dose of atorvastatin to 80 mg in further reducing total-C (-17% vs. -7%), LDL-C (-27% vs. -11%), Apo B (-18% vs. -8%), TG (-12% vs. -6%), and non-HDL-C (-23% vs. -9%). Results for HDL-C between the two treatment groups were not significantly different. Also, significantly more patients receiving ATOZET 10/40 attained LDL-C <1.8 mmol/L compared to those receiving atorvastatin 80 mg, 74% vs. 32%.
In a placebo-controlled, 8-week study, 308 hypercholesterolaemic patients receiving atorvastatin and not at National Cholesterol Education Program (NCEP) LDL-C goal (LDL-C goal based upon baseline LDL-C and CHD risk status) were randomised to receive either ezetimibe 10 mg or placebo in addition to their on-going atorvastatin therapy.
Among patients not at LDL-C goal at baseline (~83%), significantly more patients receiving ezetimibe co-administered with atorvastatin achieved their LDL-C goal compared to patients receiving placebo co-administered with atorvastatin, 67% vs. 19%. Ezetimibe added to atorvastatin therapy lowered LDL-C significantly more than placebo added to atorvastatin therapy, 25% vs. 4%. Ezetimibe added to atorvastatin therapy also significantly decreased total-C, Apo B, and TG compared with placebo added to atorvastatin therapy.
In a controlled, 12-week, 2-phase study, 1,539 high-cardiovascular-risk patients, with a LDL-C level between 2.6 and 4.1 mmol/L, on atorvastatin 10 mg daily were randomised to receive: atorvastatin 20 mg, rosuvastatin 10 mg, or ATOZET 10/10. After 6 weeks of treatment (Phase I), patients taking atorvastatin 20 mg who failed to achieve a LDL-C level <2.6 mmol/L were switched to either atorvastatin 40 mg or ATOZET 10/20 for 6 weeks (Phase II), and similar patients taking rosuvastatin 10 mg during Phase I were switched to either rosuvastatin 20 mg or ATOZET 10/20. Reductions in LDL-C and comparisons between the ATOZET group and other treatment groups studied are shown in Table 5.
Table 5. Response to ATOZET* in High Risk Patients with a LDL-C Level Between 2.6 and 4.1 mmol/L on Atorvastatin 10 mg Daily at Baseline:
| Treatment | N | Percent Change from Baseline† | |||||
|---|---|---|---|---|---|---|---|
| Total-C | LDL-C | Apo B | TG‡ | HDL-C | Non-HDL-C | ||
| Phase I | |||||||
| Switched from atorvastatin 10 mg | |||||||
| ATOZET 10/10 | 120 | -13.5 | -22.2 | -11.3 | -6.0 | +0.6 | -18.3 |
| Atorvastatin 20 mg | 480 | -6.4§ | -9.5§ | -6.0¶ | -3.9 | -1.1 | -8.1§ |
| Rosuvastatin 10 mg | 939 | -7.7§ | -13.0§ | -6.9# | -1.1 | +1.1 | -10.6§ |
| Phase II | |||||||
| Switched from atorvastatin 20 mg | |||||||
| ATOZET 10/20 | 124 | -10.7 | -17.4 | -9.8 | -5.9 | +0.7 | -15.1 |
| Atorvastatin 40 mg | 124 | -3.8Þ | -6.9Þ | -5.4 | -3.1 | +1.7 | -5.8Þ |
| Switched from rosuvastatin 10 mg | |||||||
| ATOZET 10/20 | 231 | -11.8 | -17.1 | -11.9 | -10.2 | +0.1 | -16.2 |
| Rosuvastatin 20 mg | 205 | -4.5Þ | -7.5Þ | -4.1Þ | -3.2ß | +0.8 | -6.4Þ |
* Co-administered ezetimibe and atorvastatin equivalent to ATOZET 10/10 or ATOZET 10/20
† M-Estimates (based on the method of Huber; 95% CI and p-value were obtained from fitting a robust Regression model with terms for treatment and baseline)
‡ Geometric mean percent changes from baseline in TG were calculated based on back-transformation via exponentiation of the model-based least square (LS) means and expressed as (geometric mean – 1) multiplied by 100
§ p<0.001 vs ATOZET 10/10
¶ p<0.01 vs ATOZET 10/10
# p<0.05 vs ATOZET 10/10
Þ p<0.001 vs ATOZET 10/20
ß p<0.05 vs ATOZET 10/20
Table 5 does not contain data comparing the effects of ATOZET 10/10 or 10/20 to doses higher than atorvastatin 40 mg or rosuvastatin 20 mg.
In a placebo-controlled study, the Myocardial Ischaemia Reduction with Aggressive Cholesterol-Lowering (MIRACL) study, patients with an acute coronary syndrome (non Q-wave MI or unstable angina) were randomised to receive atorvastatin 80 mg/day (n=1,538) or placebo (n=1,548). Treatment was initiated during the acute phase after hospital admission and lasted for 16 weeks. Atorvastatin 80 mg/day provided a 16% (p=0.048) reduction in risk of the combined primary endpoint: death from any cause, nonfatal MI, resuscitated cardiac arrest, or angina pectoris with evidence of myocardial ischaemia requiring hospitalisation. This was mainly due to a 26% reduction in re-hospitalisation for angina pectoris with evidence of myocardial ischaemia (p=0.018).
ATOZET contains atorvastatin. In a placebo-controlled study, the Anglo-Scandinavian Cardiac Outcomes Trial Lipid-Lowering Arm (ASCOT-LLA), the effect of atorvastatin 10 mg on fatal and non-fatal CHD was assessed in 10,305 hypertensive patients, 40-80 years old, with TC levels ≤6.5 mmol/L and at least three cardiovascular risk factors. Patients were followed for a median duration of 3.3 years. Atorvastatin 10 mg significantly (p<0.001) reduced the relative risk for: fatal CHD plus nonfatal MI by 36% (absolute risk reduction = 1.1%); total cardiovascular events and revascularisation procedures by 20% (absolute risk reduction = 1.9%); and total coronary events by 29% (absolute risk reduction = 1.4%).
In a placebo-controlled study, the Collaborative Atorvastatin Diabetes Study (CARDS), the effect of atorvastatin 10 mg on cardiovascular disease (CVD) endpoints was assessed in 2838 patients, 40-75 years old, with type 2 diabetes, one or more cardiovascular risk factors, LDL ≤4.1 mmol/L, and TG ≤6.8 mmol/L. Patients were followed for a median duration of 3.9 years. Atorvastatin 10 mg significantly (p<0.05) reduced: the rate of major cardiovascular events by 37% (absolute risk reduction = 3.2%); the risk of stroke by 48% (absolute risk reduction = 1.3%); and the risk of MI by 42% (absolute risk reduction = 1.9%).
Prevention of Cardiovascular Events
In an ezetimibe/simvastatin, multicentre, randomised, double-blind, active-control study, 18,144 patients enrolled within 10 days of hospitalisation for acute coronary syndrome (ACS; either acute myocardial infarction [MI] or unstable angina [UA]). All patients were randomised in a 1:1 ratio to receive either ezetimibe/simvastatin 10/40 mg (n=9,067) or simvastatin 40 mg (n=9,077) and followed for a median of 6.0 years.
Patients had a mean age of 63.6 years; 76% were male, 84% were Caucasian, and 27% were diabetic. The average LDL-C value at the time of study qualifying event was 80 mg/dL (2.1 mmol/L) for those on lipid-lowering therapy (n=6,390) and 101 mg/dL (2.6 mmol/L) for those not on previous lipidlowering therapy (n=11,594). Prior to the hospitalisation for the qualifying ACS event, 34% of the patients were on statin therapy. At one-year, the average LDL-C for patients continuing on therapy was 53.2 mg/dL (1.4 mmol/L) for the ezetimibe/simvastatin group and 69.9 mg/dL (1.8 mmol/L) for the simvastatin monotherapy group.
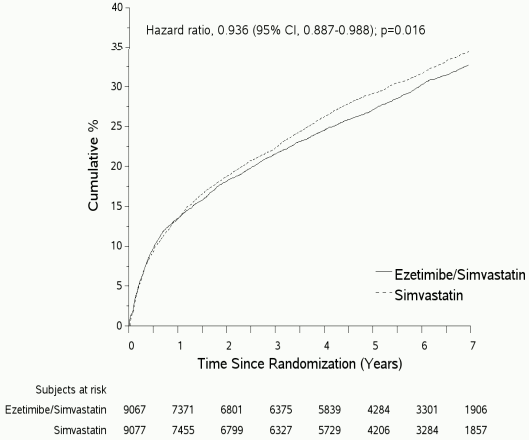
The primary endpoint was a composite consisting of cardiovascular death, major coronary events (MCE; defined as non-fatal myocardial infarction, documented unstable angina that required hospitalisation, or any coronary revascularisation procedure occurring at least 30 days after randomised treatment assignment) and non-fatal stroke. The study demonstrated that treatment with ezetimibe/simvastatin provided incremental benefit in reducing the primary composite endpoint of cardiovascular death, MCE, and non-fatal stroke compared with simvastatin alone (relative risk reduction of 6.4%, p=0.016). The primary endpoint occurred in 2,572 of 9,067 patients (7-year Kaplan-Meier [KM] rate 32.72%) in the ezetimibe/simvastatin group and 2,742 of 9,077 patients (7-year KM rate 34.67%) in the simvastatin alone group. (See Figure 1 and Table 6.) This incremental benefit is expected to be similar with co-administration of ezetimibe and atorvastatin. Total mortality was unchanged in this high risk group.
There was an overall benefit for all strokes; however there was a small non-significant increase in haemorrhagic stroke in the ezetimibe-simvastatin group compared with simvastatin alone. The risk of haemorrhagic stroke for ezetimibe co-administered with higher potency statins in long-term outcome studies has not been evaluated.
The treatment effect of ezetimibe/simvastatin was generally consistent with the overall results across many subgroups, including sex, age, race, medical history of diabetes mellitus, baseline lipid levels, prior statin therapy, prior stroke, and hypertension.
Figure 1. Effect of ezetimibe/simvastatin on the Primary Composite Endpoint of Cardiovascular Death, Major Coronary Event, or Non-fatal Stroke:
Table 6. Major Cardiovascular Events by Treatment Group in All Randomised Patients in IMPROVEIT:
| Outcome | Ezetimibe/Simvastatin 10/40 mg* (N=9,067) | Simvastatin 40 mg† (N=9,077) | Hazard Ratio (95% CI) | p-value | ||
|---|---|---|---|---|---|---|
| n | K-M %‡ | n | K-M %‡ | |||
| Primary Composite Efficacy Endpoint | ||||||
| (CV death, Major Coronary Events and non-fatal stroke) | 2,572 | 32.72% | 2,742 | 34.67% | 0.936 (0.887, 0.988) | 0.016 |
| Components of Primary Composite Endpoint and Select Efficacy Endpoints (first occurrences of specified event at any time) | ||||||
| Cardiovascular death | 537 | 6.89% | 538 | 6.84% | 1.000 (0.887, 1.127) | 0.997 |
| Major Coronary Event: | ||||||
| Non-fatal MI | 945 | 12.77% | 1,083 | 14.41% | 0.871 (0.798, 0.950) | 0.002 |
| Unstable angina requiring hospitalisation | 156 | 2.06% | 148 | 1.92% | 1.059 (0.846, 1.326) | 0.618 |
| Coronary revascularisation after 30 days | 1,690 | 21.84% | 1,793 | 23.36% | 0.947 (0.886, 1.012) | 0.107 |
| Non-fatal stroke | 245 | 3.49% | 305 | 4.24% | 0.802 (0.678, 0.949) | 0.010 |
* 6% were uptitrated to ezetimibe/simvastatin 10/80 mg
† 27% were uptitrated to simvastatin 80 mg
‡ Kaplan-Meier estimate at 7 years
Homozygous Familial Hypercholesterolaemia (HoFH)
A double-blind, randomised, 12-week study was performed in patients with a clinical and/or genotypic diagnosis of HoFH. Data were analysed from a subgroup of patients (n=36) receiving atorvastatin 40 mg at baseline. Increasing the dose of atorvastatin from 40 to 80 mg (n=12) produced a reduction of LDL-C of 2% from baseline on atorvastatin 40 mg. Co-administered ezetimibe and atorvastatin equivalent to ATOZET (10/40 and 10/80 pooled, n=24), produced a reduction of LDL-C of 19% from baseline on atorvastatin 40 mg. In those patients co-administered ezetimibe and atorvastatin equivalent to ATOZET (10/80, n=12), a reduction of LDL-C of 25% from baseline on atorvastatin 40 mg was produced.
After completing the 12-week study, eligible patients (n=35), who were receiving atorvastatin 40 mg at baseline, were assigned to co-administered ezetimibe and atorvastatin equivalent to ATOZET 10/40 for up to an additional 24 months. Following at least 4 weeks of treatment, the atorvastatin dose could be doubled to a maximum dose of 80 mg. At the end of the 24 months, ATOZET (10/40 and 10/80 pooled) produced a reduction of LDL-C that was consistent with that seen in the 12-week study.
The European Medicines Agency has waived the obligation to submit the results of studies with ATOZET in all subsets of the paediatric population in the treatments of hypercholesterolaemia and mixed hyperlipidaemia (see section 4.2 for information on paediatric use).
5.2. Pharmacokinetic properties
ATOZET
ATOZET has been shown to be bioequivalent to co-administration of corresponding doses of ezetimibe and atorvastatin tablets.
Absorption
ATOZET
The effects of a high-fat meal on the pharmacokinetics of ezetimibe and atorvastatin when administered as ATOZET tablets are comparable to those reported for the individual tablets.
Ezetimibe
After oral administration, ezetimibe is rapidly absorbed and extensively conjugated to a pharmacologically active phenolic glucuronide (ezetimibe-glucuronide). Mean maximum plasma concentrations (Cmax) occur within 1 to 2 hours for ezetimibe-glucuronide and 4 to 12 hours for ezetimibe. The absolute bioavailability of ezetimibe cannot be determined as the compound is virtually insoluble in aqueous media suitable for injection.
Concomitant food administration (high-fat or non-fat meals) had no effect on the oral bioavailability of ezetimibe when administered as 10-mg tablets.
Atorvastatin
Atorvastatin is rapidly absorbed after oral administration; maximum plasma concentrations (Cmax) occur within 1 to 2 hours. Extent of absorption increases in proportion to atorvastatin dose. After oral administration, atorvastatin film-coated tablets are 95% to 99% bioavailable compared to the oral solution. The absolute bioavailability of atorvastatin is approximately 12% and the systemic availability of HMG-CoA reductase inhibitory activity is approximately 30%. The low systemic availability is attributed to presystemic clearance in gastrointestinal mucosa and/or hepatic first-pass metabolism.
Distribution
Ezetimibe
Ezetimibe and ezetimibe-glucuronide are bound 99.7% and 88 to 92% to human plasma proteins, respectively.
Atorvastatin
Mean volume of distribution of atorvastatin is approximately 381 l. Atorvastatin is ≥98% bound to plasma proteins.
Biotransformation
Ezetimibe
Ezetimibe is metabolised primarily in the small intestine and liver via glucuronide conjugation (a phase II reaction) with subsequent biliary excretion. Minimal oxidative metabolism (a phase I reaction) has been observed in all species evaluated. Ezetimibe and ezetimibe-glucuronide are the major drug-derived compounds detected in plasma, constituting approximately 10 to 20% and 80 to 90% of the total drug in plasma, respectively. Both ezetimibe and ezetimibe-glucuronide are slowly eliminated from plasma with evidence of significant enterohepatic recycling. The half-life for ezetimibe and ezetimibe-glucuronide is approximately 22 hours.
Atorvastatin
Atorvastatin is metabolised by cytochrome P450 3A4 to ortho- and parahydroxylated derivatives and various beta-oxidation products. Apart from other pathways these products are further metabolised via glucuronidation. In vitro, inhibition of HMG-CoA reductase by ortho- and parahydroxylated metabolites is equivalent to that of atorvastatin. Approximately 70% of circulating inhibitory activity for HMG-CoA reductase is attributed to active metabolites.
Elimination
Ezetimibe
Following oral administration of 14C-ezetimibe (20 mg) to human subjects, total ezetimibe accounted for approximately 93% of the total radioactivity in plasma. Approximately 78% and 11% of the administered radioactivity were recovered in the faeces and urine, respectively, over a 10-day collection period. After 48 hours, there were no detectable levels of radioactivity in the plasma.
Atorvastatin
Atorvastatin is eliminated primarily in bile following hepatic and/or extrahepatic metabolism. However, the medicinal product does not appear to undergo significant enterohepatic recirculation. Mean plasma elimination half-life of atorvastatin in humans is approximately 14 hours. The half-life of inhibitory activity for HMG-CoA reductase is approximately 20 to 30 hours due to the contribution of active metabolites.
Atorvastatin is a substrate of the hepatic transporters, organic anion-transporting polypeptide 1B1 (OATP1B1) and 1B3 (OATP1B3) transporter. Metabolites of atorvastatin are substrates of OATP1B1. Atorvastatin is also identified as a substrate of the efflux transporters multi-drug resistance protein 1 (MDR1) and breast cancer resistance protein (BCRP), which may limit the intestinal absorption and biliary clearance of atorvastatin.
Paediatric population
Ezetimibe
The pharmacokinetics of ezetimibe are similar between children ≥ 6 years and adults. Pharmacokinetic data in the paediatric population <6 years of age are not available. Clinical experience in paediatric and adolescent patients includes patients with HoFH, HeFH, or sitosterolaemia.
Atorvastatin
In an open-label, 8-week study, Tanner Stage 1 (n=15) and Tanner Stage 2 (n=24) paediatric patients (ages 6-17 years) with heterozygous familial hypercholesterolaemia and baseline LDL-C ≥4 mmol/L were treated with 5 or 10 mg of chewable or 10 or 20 mg of film-coated atorvastatin tablets once daily, respectively. Body weight was the only significant covariate in atorvastatin population PK model. Apparent oral clearance of atorvastatin in paediatric subjects appeared similar to adults when scaled allometrically by body weight. Consistent decreases in LDL-C and TC were observed over the range of atorvastatin and o-hydroxyatorvastatin exposures.
Elderly
Ezetimibe
Plasma concentrations for total ezetimibe are about 2-fold higher in the elderly (≥65 years) than in the young (18 to 45 years). LDL-C reduction and safety profile are comparable between elderly and younger subjects treated with ezetimibe.
Atorvastatin
Plasma concentrations of atorvastatin and its active metabolites are higher in healthy elderly subjects than in young adults while the lipid effects were comparable to those seen in younger patient populations.
Hepatic impairment
Ezetimibe
After a single 10-mg dose of ezetimibe, the mean AUC for total ezetimibe was increased approximately 1.7-fold in patients with mild hepatic insufficiency (Child-Pugh score 5 or 6), compared to healthy subjects. In a 14-day, multiple-dose study (10 mg daily) in patients with moderate hepatic insufficiency (Child-Pugh score 7 to 9), the mean AUC for total ezetimibe was increased approximately 4-fold on Day 1 and Day 14 compared to healthy subjects. No dose adjustment is necessary for patients with mild hepatic insufficiency. Due to the unknown effects of the increased exposure to ezetimibe in patients with moderate or severe (Child-Pugh score >9) hepatic insufficiency, ezetimibe is not recommended in these patients (see sections 4.2 and 4.4).
Atorvastatin
Plasma concentrations of atorvastatin and its active metabolites are markedly increased (approx. 16-fold in Cmax and approx. 11-fold in AUC) in patients with chronic alcoholic liver disease (Child-Pugh B).
Renal impairment
Ezetimibe
After a single 10-mg dose of ezetimibe in patients with severe renal disease (n=8; mean CrCl ≤30 mL/min/1.73 m²), the mean AUC for total ezetimibe was increased approximately 1.5-fold, compared to healthy subjects (n=9).
An additional patient in this study (post-renal transplant and receiving multiple medicinal products, including ciclosporin) had a 12-fold greater exposure to total ezetimibe.
Atorvastatin
Renal disease has no influence on the plasma concentrations or lipid effects of atorvastatin and its active metabolites.
Gender
Ezetimibe
Plasma concentrations for total ezetimibe are slightly higher (approximately 20%) in women than in men. LDL-C reduction and safety profile are comparable between men and women treated with ezetimibe.
Atorvastatin
Concentrations of atorvastatin and its active metabolites in women differ from those in men (women: approx. 20% higher for Cmax and approx. 10% lower for AUC). These differences were of no clinical significance, resulting in no clinically significant differences in lipid effects among men and women.
SLCO1B1 polymorphism
Atorvastatin
Hepatic uptake of all HMG-CoA reductase inhibitors, including atorvastatin, involves the OATP1B1 transporter. In patients with SLCO1B1 polymorphism there is a risk of increased exposure of atorvastatin, which may lead to an increased risk of rhabdomyolysis (see section 4.4). Polymorphism in the gene encoding OATP1B1 (SLCO1B1 c.521CC) is associated with a 2.4-fold higher atorvastatin exposure (AUC) than in individuals without this genotype variant (c.521TT). A genetically impaired hepatic uptake of atorvastatin is also possible in these patients. Possible consequences for the efficacy are unknown.
5.3. Preclinical safety data
ATOZET
In three-month co-administration studies in rats and dogs with ezetimibe and atorvastatin, the toxic effects observed were essentially those typically associated with statins. The statin-like histopathologic findings were limited to the liver. Some of the toxic effects were more pronounced than those observed during treatment with statins alone. This is attributed to pharmacokinetic and/or pharmacodynamic interactions following co-administration.
The co-administration of ezetimibe and atorvastatin in pregnant rats indicated that there was a test article-related increase in the skeletal variation "reduced ossification of the sternebrae" in the high dose (1,000/108.6 mg/kg) ezetimibe/atorvastatin group. This may be related to the observed decrease in foetal body weights. In pregnant rabbits a low incidence of skeletal deformities (fused sternebrae, fused caudal vertebrae and asymmetrical sternebrae variation) were observed.
In a series of in vivo and in vitro assays, ezetimibe, given alone or co-administered with atorvastatin, exhibited no genotoxic potential.
Ezetimibe
Animal studies on the chronic toxicity of ezetimibe identified no target organs for toxic effects. In dogs treated for four weeks with ezetimibe (≥0.03 mg/kg/day) the cholesterol concentration in the cystic bile was increased by a factor of 2.5 to 3.5. However, in a one-year study on dogs given doses of up to 300 mg/kg/day no increased incidence of cholelithiasis or other hepatobiliary effects were observed. The significance of these data for humans is not known. A lithogenic risk associated with the therapeutic use of ezetimibe cannot be ruled out.
Long-term carcinogenicity tests on ezetimibe were negative.
Ezetimibe had no effect on the fertility of male or female rats, nor was it found to be teratogenic in rats or rabbits, nor did it affect prenatal or postnatal development. Ezetimibe crossed the placental barrier in pregnant rats and rabbits given multiple doses of 1,000 mg/kg/day.
Atorvastatin
Atorvastatin was negative for mutagenic and clastogenic potential in a battery of 4 in vitro tests and 1 in vivo assay. Atorvastatin was not found to be carcinogenic in rats, but high doses in mice (resulting in 6-11-fold the AUC0-24h reached in humans at the highest recommended dose) showed hepatocellular adenomas in males and hepatocellular carcinomas in females. There is evidence from animal experimental studies that HMG-CoA reductase inhibitors may affect the development of embryos or foetuses. In rats, rabbits, and dogs atorvastatin had no effect on fertility and was not teratogenic, however, at maternally toxic doses foetal toxicity was observed in rats and rabbits. The development of the rat offspring was delayed and post-natal survival reduced during exposure of the dams to high doses of atorvastatin. In rats, there is evidence of placental transfer. In rats, plasma concentrations of atorvastatin are similar to those in milk. It is not known whether atorvastatin or its metabolites are excreted in human milk.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.