ATROVENT HFA Inhalation aerosol, metered Ref.[10538] Active ingredients: Ipratropium
Source: FDA, National Drug Code (US) Revision Year: 2020
12.1. Mechanism of Action
Ipratropium bromide is an anticholinergic (parasympatholytic) agent which, based on animal studies, appears to inhibit vagally-mediated reflexes by antagonizing the action of acetylcholine, the transmitter agent released at the neuromuscular junctions in the lung. Anticholinergics prevent the increases in intracellular concentration of Ca++ which is caused by interaction of acetylcholine with the muscarinic receptors on bronchial smooth muscle.
12.2. Pharmacodynamics
Cardiovascular effects
At recommended doses, ipratropium bromide does not produce clinically significant changes in pulse rate or blood pressure.
Ocular effects
In studies without a positive control, ipratropium bromide did not alter pupil size, accommodation, or visual acuity.
Mucociliary clearance and respiratory secretions
Controlled clinical studies have demonstrated that ipratropium bromide does not alter either mucociliary clearance or the volume or viscosity of respiratory secretions.
12.3. Pharmacokinetics
Following administration by oral inhalation from a metered-dose inhaler, the majority of the delivered dose is deposited in the gastrointestinal tract and, to a lesser extent, in the lung, the intended site of action. Ipratropium bromide is a quaternary amine and hence is not readily absorbed into the systemic circulation either from the surface of the lung or from the gastrointestinal tract as confirmed by blood level and renal excretion studies.
The half-life of elimination is about 2 hours after inhalation or intravenous administration. Ipratropium bromide is minimally bound (0% to 9% in vitro) to plasma albumin and α1-acid glycoprotein. It is partially metabolized to inactive ester hydrolysis products. Following intravenous administration, approximately one-half of the dose is excreted unchanged in the urine.
A pharmacokinetic study with 29 chronic obstructive pulmonary disease (COPD) patients (48-79 years of age) demonstrated that mean peak plasma ipratropium concentrations of 59±20 pg/mL were obtained following a single administration of 4 inhalations of ATROVENT HFA (84 mcg). Plasma ipratropium concentrations declined to 24±15 pg/mL by six hours. When these patients were administered 4 inhalations QID (16 inhalations/day=336 mcg) for one week, the mean peak plasma ipratropium concentration increased to 82±39 pg/mL with a trough (6 hour) concentration of 28±12 pg/mL at steady state.
Specific Populations
Geriatric Patients
In the pharmacokinetic study with 29 COPD patients, a subset of 14 patients were >65 years of age. Mean peak plasma ipratropium concentrations of 56±24 pg/mL were obtained following a single administration of 4 inhalations (21 mcg/puff) of ATROVENT HFA (84 mcg). When these 14 patients were administered 4 inhalations four times a day (16 inhalations/day) for one week, the mean peak plasma ipratropium concentration only increased to 84±50 pg/mL indicating that the pharmacokinetic behavior of ipratropium bromide in the geriatric population is consistent with younger patients.
Renally Impaired Patients
The pharmacokinetics of ATROVENT HFA have not been studied in patients with renal insufficiency.
Hepatically Impaired Patients
The pharmacokinetics of ATROVENT HFA have not been studied in patients with hepatic insufficiency.
Drug-Drug Interaction
No specific pharmacokinetic studies were conducted to evaluate potential drug-drug interactions with other medications.
13.1. Carcinogenesis, Mutagenesis, Impairment of Fertility
Two-year oral carcinogenicity studies in rats and mice have revealed no carcinogenic activity at doses up to 6 mg/kg (approximately 240 and 120 times the maximum recommended human daily inhalation dose (MRHDID) in adults on a mg/m² basis, respectively). Results of various mutagenicity/clastogenicity studies (Ames test, mouse dominant lethal test, mouse micronucleus test and chromosome aberrations of bone marrow in Chinese hamsters) were negative.
Fertility of male or female rats at oral doses up to 50 mg/kg (approximately 2000 times the MRHDID in adults on a mg/m² basis) was unaffected by ipratropium bromide administration. At an oral dose of 500 mg/kg (approximately 20,000 times the MRHDID in adults on a mg/m² basis), ipratropium bromide produced a decrease in the conception rate.
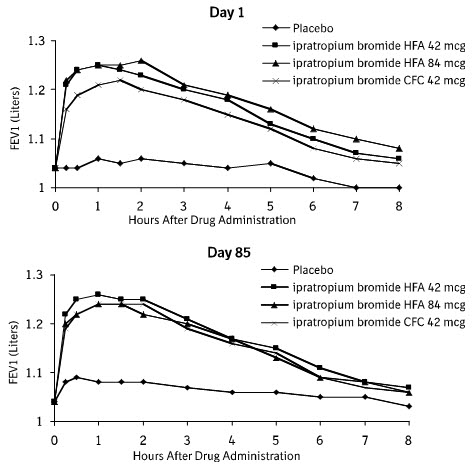
14. Clinical Studies
Conclusions regarding the efficacy of ATROVENT HFA were derived from two randomized, double-blind, controlled clinical studies. These studies enrolled males and females ages 40 years and older, with a history of COPD, a smoking history of >10 pack-years, an FEV1 <65% and an FEV1/FVC <70%.
One of the studies was a 12-week randomized, double-blind active, and placebo-controlled study in which 505 of the 507 randomized COPD patients were evaluated for the safety and efficacy of 42 mcg (n=124) and 84 mcg (n=126) ATROVENT HFA in comparison to 42 mcg (n=127) ATROVENT CFC and their respective placebos (HFA n=62, CFC n=66). Data for both placebo HFA and placebo CFC were combined in the evaluation.
Serial FEV1 (shown in Figure 1, below, as means adjusted for center and baseline effects on test day 1 and test day 85 (primary endpoint)) demonstrated that 1 dose (2 inhalations/21 mcg each) of ATROVENT HFA produced significantly greater improvement in pulmonary function than placebo. During the six hours immediately post-dose on day 1, the average hourly improvement in adjusted mean FEV1 was 0.148 liters for ATROVENT HFA (42 mcg) and 0.013 liters for placebo. The mean peak improvement in FEV1, relative to baseline, was 0.295 liters, compared to 0.138 liters for placebo. During the six hours immediately post-dose on day 85, the average hourly improvement in adjusted mean FEV1 was 0.141 liters for ATROVENT HFA (42 mcg) and 0.014 liters for placebo. The mean peak improvement in FEV1, relative to baseline, was 0.295 liters, compared to 0.140 liters for placebo.
ATROVENT HFA (42 mcg) was shown to be clinically comparable to ATROVENT CFC (42 mcg).
Figure 1. Day 1 and Day 85 (Primary Endpoint) Results:
In this study, both ATROVENT HFA and ATROVENT CFC formulations were equally effective in patients over 65 years of age and under 65 years of age.
The median time to improvement in pulmonary function (FEV1 increase of 15% or more) was within approximately 15 minutes, reached a peak in 1 to 2 hours, and persisted for 2 to 4 hours in the majority of the patients. Improvements in Forced Vital Capacity (FVC) were also demonstrated.
The other study was a 12-week, randomized, double-blind, active-controlled clinical study in 174 adults with COPD, in which ATROVENT HFA 42 mcg (n=118) was compared to ATROVENT CFC 42 mcg (n=56). Safety and efficacy of HFA and CFC formulations were shown to be comparable.
The bronchodilatory efficacy and comparability of ATROVENT HFA vs ATROVENT CFC were also studied in a one-year open-label safety and efficacy study in 456 COPD patients. The safety and efficacy of HFA and CFC formulations were shown to be comparable.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.