AURYXIA Film-coated tablet Ref.[10060] Active ingredients: Ferric citrate
Source: FDA, National Drug Code (US) Revision Year: 2019
Product description
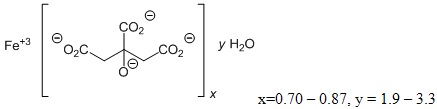
Auryxia (ferric citrate), a phosphate binder and iron replacement product, is known chemically as iron (+3), x (1, 2, 3-propanetricarboxylic acid, 2 hydroxy-), y (H2O)
Auryxia 210 mg ferric iron tablets for oral administration, equivalent to 1g ferric citrate, are film-coated, peach-colored, and oval-shaped tablets debossed with “KX52”. The inactive ingredients are pregelatinized starch and calcium stearate. In addition, the film-coating contains the following inactive ingredients; hypromellose, titanium dioxide, triacetin, and FD&C Yellow #6/Sunset Yellow FCF Aluminum Lake, FD&C Red #40/Allura Red AC Aluminum Lake, and FD&C Blue #2/Indigo Carmine Aluminum Lake.
| Dosage Forms and Strengths |
|---|
|
Tablets: Auryxia 210 mg ferric iron, equivalent to 1 g ferric citrate, film-coated, peach-colored, and oval-shaped tablet debossed with “KX52”. |
| How Supplied |
|---|
|
Tablets: Auryxia 210 mg ferric iron tablets equivalent to 1 g of ferric citrate are supplied as 200 tablets in 400-cc high-density polyethylene bottles. The 210 mg ferric iron tablets are film-coated, peach-colored, and oval-shaped tablets debossed with "KX52." 1 Bottle of 200-count 210 mg ferric iron tablets (NDC 59922-631-01) |
Drugs
| Drug | Countries | |
|---|---|---|
| AURYXIA | United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.