BAFIERTAM Capsule Ref.[10345] Active ingredients:
Source: FDA, National Drug Code (US) Revision Year: 2020
Product description
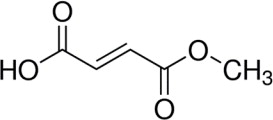
BAFIERTAM contains the active ingredient monomethyl fumarate, which is an unsaturated monomethyl ester. It is also known by its chemical name, fumaric acid monomethyl ester, (C5H6O4). It has the following structure:
Monomethyl fumarate is a white to off-white powder that is highly soluble in water with a molecular mass of 130.10.
BAFIERTAM is provided as soft gelatin delayed-release capsules for oral administration, containing
95 mg of monomethyl fumarate consisting of the following inactive ingredients: Glyceryl caprylate/caprate; lactic acid; polyoxyl 40 hydrogenated castor oil; and povidone K30.The capsule shell, printed with black ink, contains the following inactive ingredients: gelatin; solution of sorbitans and sorbitol; and titanium dioxide. The coating system includes the following inactive ingredients: colloidal anhydrous silica, GMCC Type 1 mono and di-glycerides, hypromellose type 2910, methacrylic acid and ethyl acrylate copolymer, polyethylene glycol (MW=400), polyvinyl alcohol part hydrolyzed, sodium bicarbonate, sodium lauryl sulfate, talc, titanium dioxide, and triethyl citrate.
| Dosage Forms and Strengths |
|---|
|
BAFIERTAM is available as soft gelatin delayed-release capsules containing 95 mg of monomethyl fumarate. The 95 mg capsule is white, opaque, oval, coated, and printed with “95” in black ink on the body. |
| How Supplied |
|---|
|
BAFIERTAM is available as soft gelatin delayed-release capsules containing 95 mg of monomethyl fumarate. The 95 mg capsules are white, opaque, oval, and coated with “95” printed in black ink on the body. BAFIERTAM is available as follows: 95 mg capsules: bottle of 120 capsules (NDC 69387-001-01). Manufactured by: Banner Life Sciences LLC, High Point, NC 27265 |
Drugs
| Drug | Countries | |
|---|---|---|
| BAFIERTAM | United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.