BASAGLAR Solution for injection Kwikpen / Tempo pen Ref.[10756] Active ingredients: Insulin glargine
Source: FDA, National Drug Code (US) Revision Year: 2020
Product description
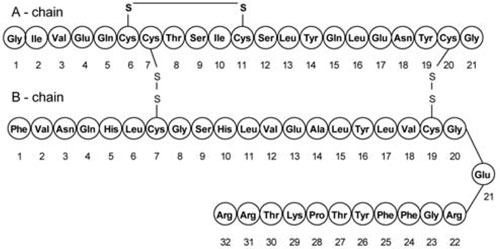
Insulin glargine injection is a long-acting insulin for subcutaneous use. Insulin glargine is a recombinant human insulin analog [see Clinical Pharmacology (12)]. BASAGLAR is produced by recombinant DNA technology utilizing a non-pathogenic laboratory strain of Escherichia coli (K12) as the production organism. Insulin glargine differs from human insulin in that the amino acid asparagine at position A21 is replaced by glycine and two arginines are added to the C-terminus of the B-chain. Chemically, insulin glargine is 21A-Gly-30B-a-L-Arg-30Bb-L-Arg-human insulin and has the empirical formula C267H404N72O78S6 and a molecular weight of 6063.
Insulin glargine has the following structural formula:
BASAGLAR (insulin glargine injection) is a clear, colorless, sterile aqueous solution for subcutaneous use. Each milliliter of BASAGLAR (insulin glargine injection) contains 100 units of insulin glargine (3.6378 mg).
The 3 mL BASAGLAR prefilled pen presentations contain the following inactive ingredients per mL: glycerin (17 mg), metacresol (2.7 mg), zinc (30 mcg), zinc oxide content adjusted to provide 0.03 mg zinc ion, and Water for Injection, USP.
The pH is adjusted by addition of aqueous solutions of hydrochloric acid 10% and/or sodium hydroxide 10%. BASAGLAR has a pH of approximately 4.
| Dosage Forms and Strengths |
|---|
|
Injection: 100 units per mL (U-100) clear, colorless, sterile solution, available as:
|
| How Supplied | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
BASAGLAR (insulin glargine injection) is a clear, colorless, sterile solution, with no visible particles, 100 units per mL (U-100) available as:
a Tempo Pen contains a component that allows for data connectivity when used with a compatible transmitter. The BASAGLAR KwikPen and Tempo Pen dial in 1 unit increments. Needles are not included. This device is recommended for use with Becton, Dickinson & Company's insulin pen needles which are sold separately. Marketed by: Lilly USA, LLC, Indianapolis, IN 46285, USA |
Drugs
| Drug | Countries | |
|---|---|---|
| BASAGLAR | Brazil, Canada, Ecuador, Hong Kong, Israel, Singapore, Turkey, United States, South Africa |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.