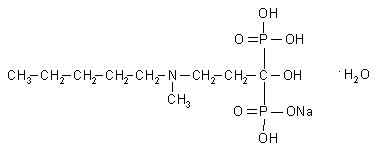BONIVA Film-coated tablet Ref.[50365] Active ingredients: Ibandronic acid
Source: FDA, National Drug Code (US) Revision Year: 2021
Product description
BONIVA (ibandronate sodium) is a nitrogen-containing bisphosphonate that inhibits osteoclast-mediated bone resorption. The chemical name for ibandronate sodium is 3-(NmethylN-pentyl) amino-1-hydroxypropane-1,1-diphosphonic acid, monosodium salt, monohydrate with the molecular formula C9H22NO7P2Na•H2O and a molecular weight of 359.24. Ibandronate sodium is a white- to off-white powder. It is freely soluble in water and practically insoluble in organic solvents.
Ibandronate sodium has the following structural formula:
BONIVA is available as a white, oblong, 150 mg film-coated tablet for once-monthly oral administration. One 150 mg film-coated tablet contains 168.75 mg ibandronate monosodium monohydrate, equivalent to 150 mg free acid. BONIVA also contains the following inactive ingredients: lactose monohydrate, povidone, microcrystalline cellulose, crospovidone, purified stearic acid, colloidal silicon dioxide, and purified water. The tablet film coating contains hypromellose, titanium dioxide, talc, polyethylene glycol 6000, and purified water.
| Dosage Forms and Strengths |
|---|
|
BONIVA 150 mg tablets: white, oblong, engraved with “BNVA” on one side and “150” on the other side. |
| How Supplied |
|---|
|
BONIVA 150 mg tablets: supplied as white, oblong, film-coated tablets, engraved with “BNVA” on one side and “150” on the other side. Packaged as a three-month supply in: A box with 1 blister pack containing 3 tablets (NDC 0004-0186-83). BONIVA is a registered trademark of Roche Therapeutics Inc. Distributed by: Genentech USA, Inc., A Member of the Roche Group, 1 DNA Way, South San Francisco, CA 94080-4990 |
Drugs
| Drug | Countries | |
|---|---|---|
| BONIVA | United States, South Africa |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.