BRALTUS Inhalation powder, hard capsule Ref.[27758] Active ingredients: Tiotropium
Source: Medicines & Healthcare Products Regulatory Agency (GB) Revision Year: 2019 Publisher: TEVA UK Limited, Brampton Road, Hampden Park, Eastbourne, East Sussex, BN22 9AG, UNITED KINGDOM
4.1. Therapeutic indications
Braltus is indicated as a maintenance bronchodilator treatment to relieve symptoms in patients with chronic obstructive pulmonary disease (COPD).
Braltus is indicated for use in adults.
4.2. Posology and method of administration
Posology
Route of administration: Inhalation use.
Recommended Dose
Adults 18 years of age and older
Inhalation of the contents of one capsule once daily with the Zonda inhaler.
Inhalation should be at the same time of day each day.
The recommended dose should not be exceeded.
The delivered dose of a single capsule (10 micrograms) is sufficient and is the standard dose for treatment with Braltus.
Braltus capsules are for inhalation only; they must not be swallowed.
Braltus capsules must only be inhaled with the Zonda inhaler.
Special populations
Elderly patients can use tiotropium bromide at the recommended dose.
Patients with mild renal impairment (creatinine clearance >50 ml/min) can use tiotropium bromide at the recommended dose. For patients with moderate to severe impairment (creatinine clearance ≤50 ml/min) see section 4.4 and section 5.2.
Hepatically impaired patients can use tiotropium bromide at the recommended dose (see section 5.2).
Paediatric population
Braltus should not be used in children or adolescents under 18 years of age. Safety and efficacy have not been established. No data are available.
There is no relevant use for tiotropium bromide in the paediatric population for the indication of COPD.
The safety and efficacy of tiotropium bromide in cystic fibrosis in children and adolescents aged less than 18 years have not been established. No data are available.
Method of administration/Instructions for Use and Handling
To ensure proper administration of the medicinal product, the patient should be trained in the use of the inhaler by either the prescribing physician or by other healthcare professionals.
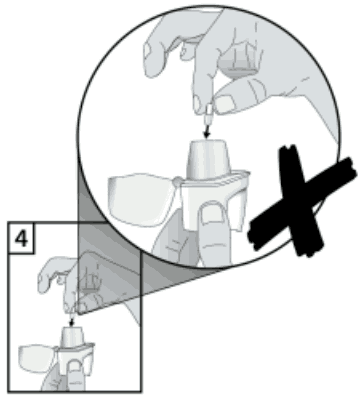
The Zonda inhaler is especially designed for Braltus capsules; patients must not use it to take any other medication. Braltus capsules must only be inhaled using the Zonda inhaler. Patients must not use any other inhalers to take Braltus capsules. Advise the patient to carefully follow the instructions for use in the patient leaflet. Make the patient aware of the additional pictures on the inside of the lid of the carton which illustrate the correct method for insertion of the capsule into the inhaler. To avoid the risk of choking, instruct the patient to NEVER place a capsule directly into the mouthpiece.
The Zonda inhaler should only be used with the bottle of capsules that will either be provided in the same pack as the inhaler, or in the pack bundled separately with the inhaler pack. Do not reuse the inhaler for another bottle of capsules. Discard the Zonda device after 30 uses (15 uses if used in conjunction with the 15 capsule presentation).
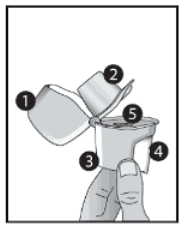
- Dust cap
- Mouthpiece
- Base
- Piercing button
- Centre chamber
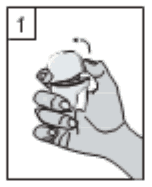
1. Pull the dust cap upwards.
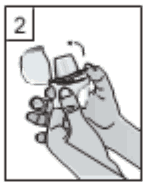
2. Hold the base of the inhaler firmly and open the mouthpiece by pulling it upwards, in the direction of the arrow to open it.
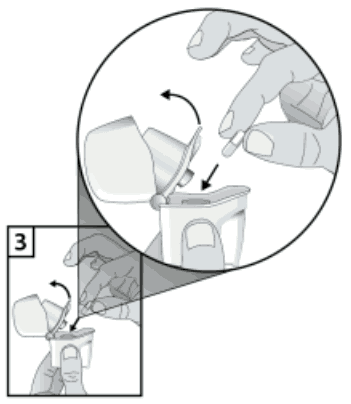
3. Remove a Braltus capsule from the bottle immediately before use and close the bottle tightly. Place one capsule in the centre chamber in the base of the inhaler. Do not store the capsule in the Zonda inhaler.
4. To avoid the risk of choking, NEVER place a capsule directly into the mouthpiece.
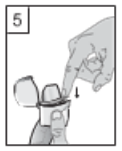
5. Close the mouthpiece until a click is heard, leaving the dust cap open.
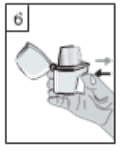
6. Hold the inhaler with the mouthpiece upwards, and press the piercing button completely in once. Release the button. This will pierce the capsule and allows the medication to be released when the patient breathes in.
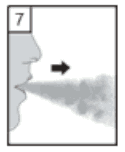
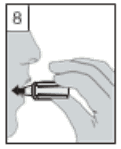
7. Breathe out fully. It is important to do this away from the mouthpiece. Avoid breathing into the mouthpiece at any time.
8. Place the mouthpiece in your mouth and keep your head in an upright position. Close your lips around the mouthpiece and breathe in slowly and deeply enough to hear or feel the capsule vibrating inside the centre chamber.
Hold your breath for as long as you comfortably can whilst taking the inhaler out of your mouth. Then breathe normally. Repeat steps 7 and 8 to empty the capsule completely.
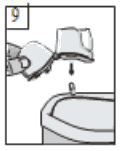
9. After use, open the mouthpiece again, and tip out the empty capsule. Close the mouthpiece and dust cap, and store the Zonda inhaler.
Braltus capsules contain only a small amount of powder, so that the capsule is only partially filled.
If necessary, the patient may wipe the mouthpiece of the Zonda inhaler after use with a dry cloth or tissue.
4.9. Overdose
High doses of tiotropium bromide may lead to anticholinergic signs and symptoms.
However, there were no systemic anticholinergic adverse effects following a single inhaled dose of up to 340 microgram tiotropium bromide in healthy volunteers. Additionally, no relevant adverse effects, beyond dry mouth, were observed following 7 day dosing of up to 170 microgram tiotropium bromide in healthy volunteers. In a multiple dose study in COPD patients with a maximum daily dose of 43 microgram tiotropium bromide over four weeks no significant undesirable effects have been observed.
Acute intoxication by inadvertent oral ingestion of tiotropium bromide capsules is unlikely due to low oral bioavailability.
6.3. Shelf life
24 months.
After first opening: 30 days (15 capsule bottle) or 60 days (30 capsule bottle).
6.4. Special precautions for storage
Keep the bottle tightly closed. Store in the original package to protect from moisture.
Do not refrigerate or freeze.
6.5. Nature and contents of container
High density polyethylene (HDPE) bottles closed with polypropylene (PP) screw-caps with polyethylene (PE) safety ring and low density polyethylene (LDPE) desiccant capsule containing silica gel. Each bottle contains 15 or 30 capsules, supplied in a carton with a Zonda inhaler.
The Zonda inhaler is a single dose inhalation device with a green body and cap and a white push button, made from acrylonitrile butadiene styrene (ABS) plastic materials and stainless steel.
Single pack containing either 15 or 30 capsules and a Zonda inhaler.
Multipacks containing either 60 capsules (2 packs of 30), and 2 Zonda inhalers or 90 capsules (3 packs of 30), and 3 Zonda inhalers.
Bundle pack: 30 capsules (bottle) in a box bundled with 1 Zonda inhaler packed in a separate box.
Not all pack sizes may be marketed.
6.6. Special precautions for disposal and other handling
Any unused medicinal product or waste material should be disposed of in accordance with local requirements.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.