BROMFED DM Syrup Ref.[27885] Active ingredients: Brompheniramine Demorphan Pseudoephedrine
Source: FDA, National Drug Code (US) Revision Year: 2021
Product description
Bromfed DM Cough Syrup is a clear, light pink syrup with a butterscotch flavor.
Each 5 mL (1 teaspoonful) contains:
Brompheniramine Maleate, USP 2 mg
Pseudoephedrine Hydrochloride, USP 30 mg
Dextromethorphan Hydrobromide, USP 10 mg
Alcohol 0.95% v/v
In a palatable, aromatic vehicle.
Inactive Ingredients: artificial butterscotch flavor, citric acid anhydrous, dehydrated alcohol, FD&C Red No. 40, glycerin, liquid sugar, methylparaben, propylene glycol, purified water and sodium benzoate. It may contain 10% citric acid solution or 10% sodium citrate solution for pH adjustment. The pH range is between 3.0 and 6.0.
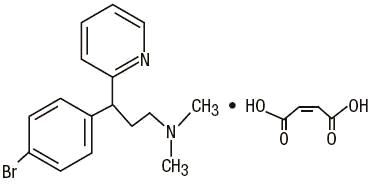
C16H19BrN2•C4H4O4 M.W. 435.31
Brompheniramine Maleate, USP (±)2pBromoα-2-(dimethylamino)ethylbenzylpyridine maleate (1:1)
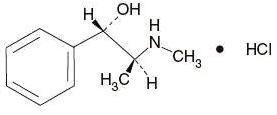
C10H15NO•HCl M.W. 201.69
Pseudoephedrine Hydrochloride, USP (+)- Pseudoephedrine hydrochloride
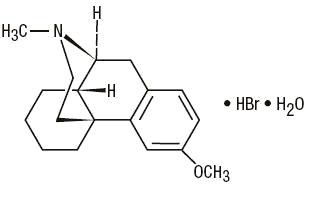
C18H25NO•HBr•H2O M.W. 370.32
Dextromethorphan Hydrobromide, USP 3-Methoxy-17-methyl-9α, 13α, 14α-morphinan hydrobromide monohydrate
Antihistamine/Nasal Decongestant/Antitussive syrup for oral administration.
| How Supplied |
|---|
|
Bromfed DM Cough Syrup is a clear, light pink-colored, butterscotch-flavored syrup containing in each 5 mL (1 teaspoonful) brompheniramine maleate 2 mg, pseudoephedrine hydrochloride 30 mg and dextromethorphan hydrobromide 10 mg, available in the following sizes: 4 fl oz (118 mL) NDC 60432-837-04 1 Pint (473 mL) NDC 60432-837-16 Manufactured For: Wockhardt USA, LLC, Parsippany, NJ 07054 Manufactured By: Morton Grove Pharmaceuticals, Inc., Morton Grove, IL 60053 |
Drugs
| Drug | Countries | |
|---|---|---|
| BROMFED DM | United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.