BUTRANS Transdermal system Ref.[10762] Active ingredients: Buprenorphine
Source: FDA, National Drug Code (US) Revision Year: 2019
12.1. Mechanism of Action
Buprenorphine is a partial agonist at the mu-opioid receptor and an antagonist at the kappa-opioid receptors, an agonist at delta-opioid receptors, and a partial agonist at ORL-1 (nociceptin) receptors. The contributions of these actions to its analgesic profile are unclear.
12.2. Pharmacodynamics
Effects on the Central Nervous System
Buprenorphine produces respiratory depression by direct action on brainstem respiratory centers. The respiratory depression involves a reduction in the responsiveness of the brainstem respiratory centers to both increases in carbon dioxide tension and electrical stimulation.
Buprenorphine causes miosis, even in total darkness. Pinpoint pupils are a sign of opioid overdose but are not pathognomonic (e.g., pontine lesions of hemorrhagic or ischemic origins may produce similar findings). Marked mydriasis rather than miosis may be seen with worsening hypoxia in overdose situations.
Effects on the Gastrointestinal Tract and Other Smooth Muscle
Buprenorphine causes a reduction in motility associated with an increase in smooth muscle tone in the antrum of the stomach and duodenum. Digestion of food in the small intestine is delayed and propulsive contractions are decreased. Propulsive peristaltic waves in the colon are decreased, while tone is increased to the point of spasm, resulting in constipation. Other opioid-induced effects may include a reduction in biliary and pancreatic secretions, spasm of sphincter of Oddi, and transient elevations in serum amylase.
Effects on the Cardiovascular System
Buprenorphine produces peripheral vasodilation, which may result in orthostatic hypotension or syncope. Manifestations of histamine release and/or peripheral vasodilation may include pruritus, flushing, red eyes, sweating, and/or orthostatic hypotension.
Effects on Cardiac Electrophysiology
The effect of BUTRANS 10 mcg/hour and 2 x BUTRANS 20 mcg/hour on QTc interval was evaluated in a double-blind (BUTRANS vs. placebo), randomized, placebo and active-controlled (moxifloxacin 400 mg, open label), parallel-group, dose-escalating, single-dose study in 132 healthy male and female subjects aged 18 to 55 years. The dose escalation sequence for BUTRANS during the titration period was: BUTRANS 5 mcg/hour for 3 days, then BUTRANS 10 mcg/hour for 3 days, then BUTRANS 20 mcg/hour for 3 days, then 2 x BUTRANS 20 mcg/hour for 4 days. The QTc evaluation was performed during the third day of BUTRANS 10 mcg/hour and the fourth day of 2 x BUTRANS 20 mcg/hour when the plasma levels of buprenorphine were at steady state for the corresponding doses [see Warnings and Precautions (5.8)].
There was no clinically meaningful effect on mean QTc with a BUTRANS dose of 10 mcg/hour. A BUTRANS dose of 40 mcg/hour (given as two 20 mcg/hour BUTRANS Transdermal Systems) prolonged mean QTc by a maximum of 9.2 (90% CI: 5.2-13.3) msec across the 13 assessment time points.
Effects on the Endocrine System
Opioids inhibit the secretion of adrenocorticotropic hormone (ACTH), cortisol, and luteinizing hormone (LH) in humans [see Adverse Reactions (6.2)]. They also stimulate prolactin, growth hormone (GH) secretion, and pancreatic secretion of insulin and glucagon.
Chronic use of opioids may influence the hypothalamic-pituitary-gonadal axis, leading to androgen deficiency that may manifest as low libido, impotence, erectile dysfunction, amenorrhea, or infertility. The causal role of opioids in the clinical syndrome of hypogonadism is unknown because the various medical, physical, lifestyle, and psychological stressors that may influence gonadal hormone levels have not been adequately controlled for in studies conducted to date [see Adverse Reactions (6.2)].
Effects on the Immune System
Opioids have been shown to have a variety of effects on components of the immune system in in vitro and animal models. The clinical significance of these findings is unknown. Overall, the effects of opioids appear to be modestly immunosuppressive.
Concentration–Efficacy Relationships
The minimum effective analgesic concentration will vary widely among patients, especially among patients who have been previously treated with potent agonist opioids. The minimum effective analgesic concentration of buprenorphine for any individual patient may increase over time due to an increase in pain, the development of a new pain syndrome, and/or the development of analgesic tolerance [see Dosage and Administration (2.1, 2.3)].
Concentration–Adverse Reaction Relationships
There is a relationship between increasing buprenorphine plasma concentration and increasing frequency of dose-related opioid adverse reactions such as nausea, vomiting, CNS effects, and respiratory depression. In opioid-tolerant patients, the situation may be altered by the development of tolerance to opioid-related adverse reactions [see Dosage and Administration (2.1, 2.2, 2.3)].
12.3. Pharmacokinetics
Absorption
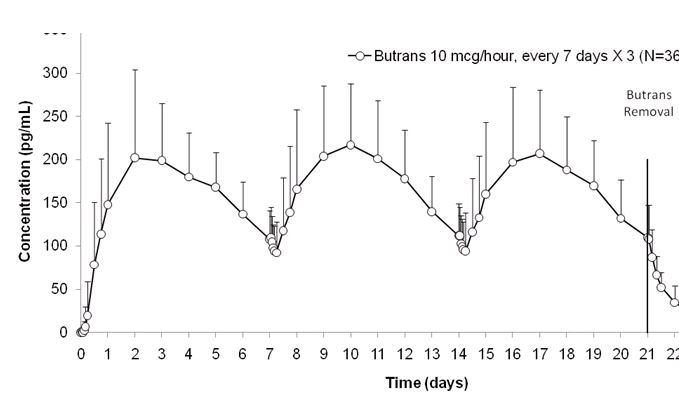
Each BUTRANS system provides delivery of buprenorphine for 7 days. Steady state was achieved during the first application by Day 3 (see Figure 2).
Figure 2. Mean (SD) Buprenorphine Plasma Concentrations Following Three Consecutive Applications of BUTRANS 10 mcg/hour (N=36 Healthy Subjects):
BUTRANS 5, 10, and 20 mcg/hour provide dose-proportional total buprenorphine exposures (AUC) following 7-day applications. BUTRANS single 7-day application and steady-state pharmacokinetic parameters are summarized in Table 7. Plasma buprenorphine concentrations after titration showed no further change over the 60-day period studied.
Table 7. Pharmacokinetic Parameters of BUTRANS in Healthy Subjects, Mean (%CV):
| Single 7-day Application | AUCinf (pg.h/mL) | Cmax (pg/mL) |
|---|---|---|
| BUTRANS 5 mcg/hour | 12087 (37) | 176 (67) |
| BUTRANS 10 mcg/hour | 27035 (29) | 191 (34) |
| BUTRANS 20 mcg/hour | 54294 (36) | 471 (49) |
| Multiple 7-day Applications | AUCtau,ss (pg.h/mL) | Cmax,ss (pg/mL) |
| BUTRANS 10 mcg/hour, steady-state | 27543 (33) | 224 (35) |
Transdermal delivery studies showed that intact human skin is permeable to buprenorphine. In clinical pharmacology studies, the median time for BUTRANS 10 mcg/hour to deliver quantifiable buprenorphine concentrations (≥ 25 pg/mL) was approximately 17 hours.
The absolute bioavailability of BUTRANS relative to IV administration, following a 7-day application, is approximately 15% for all doses (BUTRANS 5, 10, and 20 mcg/hour).
Effects of Application Site
A study in healthy subjects demonstrated that the pharmacokinetic profile of buprenorphine delivered by BUTRANS 10 mcg/hour is similar when applied to the upper outer arm, upper chest, upper back, or the side of the chest [see Dosage and Administration (2.6)].
The reapplication of BUTRANS 10 mcg/hour after various rest periods to the same application site in healthy subjects showed that the minimum rest period needed to avoid variability in drug absorption is 3 weeks (21 days) [see Dosage and Administration (2.6)].
Effects of Heat
In a study of healthy subjects, application of a heating pad directly on the BUTRANS 10 mcg/hour system caused a 26% - 55% increase in blood concentrations of buprenorphine. Concentrations returned to normal within 5 hours after the heat was removed. For this reason, instruct patients not to apply heating pads directly to the BUTRANS system during system wear [see Warnings and Precautions (5.15)].
Fever may increase the permeability of the skin, leading to increased buprenorphine concentrations during BUTRANS treatment. As a result, febrile patients are at increased risk for the possibility of BUTRANS-related reactions during treatment with BUTRANS. Monitor patients with febrile illness for adverse effects and consider dose adjustment [see Warnings and Precautions (5.15)]. In a crossover study of healthy subjects receiving endotoxin or placebo challenge during BUTRANS 10 mcg/hour wear, the AUC and Cmax were similar despite a physiologic response of mild fever to endotoxin.
Distribution
Buprenorphine is approximately 96% bound to plasma proteins, mainly to alpha- and beta-globulin.
Studies of IV buprenorphine have shown a large volume of distribution (approximately 430 L), implying extensive distribution of buprenorphine.
CSF buprenorphine concentrations appear to be approximately 15-25% of concurrent plasma concentrations.
Elimination
Metabolism
Buprenorphine metabolism in the skin following BUTRANS application is negligible.
Buprenorphine primarily undergoes N-dealkylation by CYP3A4 to norbuprenorphine and glucuronidation by UGT-isoenzymes (mainly UGT1A1 and 2B7) to buprenorphine 3β-O-glucuronide. Norbuprenorphine, the major metabolite, is also glucuronidated (mainly UGT1A3) prior to excretion.
Norbuprenorphine is the only known active metabolite of buprenorphine. It has been shown to be a respiratory depressant in rats, but only at concentrations at least 50-fold greater than those observed following application to humans of BUTRANS 20 mcg/hour.
Excretion
Following IV administration, buprenorphine and its metabolites are secreted into bile and excreted in urine.
Following intramuscular administration of 2 mcg/kg dose of buprenorphine, approximately 70% of the dose was excreted in feces within 7 days. Approximately 27% was excreted in urine.
Following transdermal application, buprenorphine is eliminated via hepatic metabolism, with subsequent biliary excretion and renal excretion of soluble metabolites. After removal of BUTRANS, mean buprenorphine concentrations decrease approximately 50% within 10-24 hours, followed by decline with an apparent terminal half-life of approximately 26 hours.
Since metabolism and excretion of buprenorphine occur mainly via hepatic elimination, reductions in hepatic blood flow induced by some general anesthetics (e.g., halothane) and other drugs may result in a decreased rate of hepatic elimination of the drug, leading to increased plasma concentrations.
The total clearance of buprenorphine is approximately 55 L/hour in postoperative patients.
Drug Interaction Studies
Effect of CYP3A4 inhibitors
In a drug-drug interaction study, BUTRANS 10 mcg/hour (single dose x 7 days) was co-administered with 200 mg ketoconazole, a strong CYP3A4 inhibitor or ketoconazole placebo twice daily for 11 days and the pharmacokinetics of buprenorphine and its metabolites were evaluated. Plasma buprenorphine concentrations did not accumulate during co-medication with ketoconazole 200 mg twice daily. Based on the results from this study, metabolism during therapy with BUTRANS is not expected to be affected by co-administration of CYP3A4 inhibitors [see Drug Interactions (7)].
Antiretroviral agents have been evaluated for CYP3A4 mediated interactions with sublingual buprenorphine. Nucleoside reverse transcriptase inhibitors (NRTIs) and non-nucleoside reverse transcriptase inhibitors (NNRTIs) do not appear to have clinically significant interactions with buprenorphine. However, certain protease inhibitors (PIs) with CYP3A4 inhibitory activity such as atazanavir and atazanavir/ritonavir resulted in elevated levels of buprenorphine and norbuprenorphine when buprenorphine and naloxone were administered sublingually. Cmax and AUC for buprenorphine increased by up to 1.6 and 1.9 fold, and Cmax and AUC for norbuprenorphine increased by up to 1.6 and 2.0 fold respectively, when sublingual buprenorphine was administered with these PIs. Patients in this study reported increased sedation, and symptoms of opiate excess have been found in post-marketing reports of patients receiving buprenorphine and atazanavir with and without ritonavir concomitantly. It should be noted that atazanavir is both a CYP3A4 and UGT1A1 inhibitor. As such, the drug-drug interaction potential for buprenorphine with CYP3A4 inhibitors is likely to be dependent on the route of administration as well as the specificity of enzyme inhibition [see Drug Interactions (7)].
Effect of CYP3A4 Inducers
The interaction between buprenorphine and CYP3A4 inducers has not been studied.
Specific Populations
Age: Geriatric Patients
Following a single application of BUTRANS 10 mcg/hour to 12 healthy young adults (mean age 32 years) and 12 healthy elderly subjects (mean age 72 years), the pharmacokinetic profile of BUTRANS was similar in healthy elderly and healthy young adult subjects, though the elderly subjects showed a trend toward higher plasma concentrations immediately after BUTRANS removal. Both groups eliminated buprenorphine at similar rates after system removal [see Use in Specific Populations (8.5)].
In a study of healthy young subjects, healthy elderly subjects, and elderly subjects treated with thiazide diuretics, BUTRANS at a fixed dose-escalation schedule (BUTRANS 5 mcg/hour for 3 days, followed by BUTRANS 10 mcg/hour for 3 days and BUTRANS 20 mcg/hour for 7 days) produced similar mean plasma concentration vs. time profiles for each of the three subject groups. There were no significant differences between groups in buprenorphine Cmax or AUC [see Use in Specific Populations (8.5)].
Sex
In a pooled data analysis utilizing data from several studies that administered BUTRANS 10 mcg/hour to healthy subjects, no differences in buprenorphine Cmax and AUC or body-weight normalized Cmax and AUC were observed between males and females treated with BUTRANS.
Hepatic Impairment
The pharmacokinetics of buprenorphine following an IV infusion of 0.3 mg of buprenorphine were compared in 8 patients with mild impairment (Child-Pugh A), 4 patients with moderate impairment (Child-Pugh B) and 12 subjects with normal hepatic function. Buprenorphine and norbuprenorphine exposure did not increase in the mild and moderate hepatic impairment patients.
BUTRANS has not been evaluated in patients with severe (Child-Pugh C) hepatic impairment [see, Warnings and Precautions (5.11), Use in Specific Populations (8.6)].
Renal Impairment
No studies in patients with renal impairment have been performed with BUTRANS.
In an independent study, the effect of impaired renal function on buprenorphine pharmacokinetics after IV bolus and after continuous IV infusion administrations was evaluated. It was found that plasma buprenorphine concentrations were similar in patients with normal renal function and in patients with impaired renal function or renal failure. In a separate investigation of the effect of intermittent hemodialysis on buprenorphine plasma concentrations in chronic pain patients with end-stage renal disease who were treated with a transdermal buprenorphine product (marketed outside the US) up to 70 mcg/hour, no significant differences in buprenorphine plasma concentrations before or after hemodialysis were observed.
No notable relationship was observed between estimated creatinine clearance rates and steady-state buprenorphine concentrations among patients during BUTRANS therapy.
13.1. Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Buprenorphine administered daily by skin painting to Sprague Dawley rats for 100 weeks at dosages (20, 60, or 200 mg/kg) produced systemic exposures (based on AUC) that ranged from approximately 130 to 350 times that of human subjects administered the maximum recommended human dose (MRHD) of BUTRANS 20 mcg/hour. An increased incidence of benign testicular interstitial cell tumors, considered buprenorphine treatment-related, was observed in male rats compared with concurrent controls. The tumor incidence was also above the highest incidence in the historical control database of the testing facility. These tumors were noted at 60 mg/kg/day and higher at approximately 220 times the proposed MRHD based on AUC. The no observed effect level (NOEL) was 20 mg/kg/day (approximately 140 times the proposed MRHD based on AUC). The mechanism leading to the tumor findings and the relevance to humans is unknown.
Buprenorphine was administered by skin painting to hemizygous Tg.AC mice over a 6-month study period. At the dosages administered daily (18.75, 37.5, 150, or 600 mg/kg/day), buprenorphine was not carcinogenic or tumorigenic at systemic exposure to buprenorphine, based on AUC, of up to approximately 1000 times that of human subjects administered BUTRANS 20 mcg/hour, the MRHD.
Mutagenesis
Buprenorphine was not genotoxic in three in vitro genetic toxicology studies (bacterial mutagenicity test, mouse lymphoma assay, chromosomal aberration assay in human peripheral blood lymphocytes), and in one in vivo mouse micronucleus test.
Impairment of Fertility
BUTRANS (¼ of a BUTRANS 5 mcg/hour, one BUTRANS 5 mcg/hour, or one BUTRANS 20 mcg/hour every 3 days in males for 4 weeks prior to mating for a total of 10 weeks and in females for 2 weeks prior to mating through Gestation Day 7) had no effect on fertility or general reproductive performance of rats at AUC-based exposure levels as high as approximately 65 times (females) and 100 times (males) that for human subjects who received BUTRANS 20 mcg/hour, the MRHD.
14. Clinical Studies
The efficacy of BUTRANS has been evaluated in four 12-week double-blind, controlled clinical trials in opioid-naïve and opioid-experienced patients with moderate to severe chronic low back pain or osteoarthritis using pain scores as the primary efficacy variable. Two of these studies, described below, demonstrated efficacy in patients with low back pain. One study in low back pain and one study in osteoarthritis did not show a statistically significant pain reduction for either BUTRANS or the respective active comparators.
12-Week Study in Opioid-Naïve Patients with Chronic Low Back Pain
A total of 1,024 patients with chronic low back pain who were suboptimally responsive to their non-opioid therapy entered an open-label, dose-titration period for up to four weeks. Patients initiated therapy with three days of treatment with BUTRANS 5 mcg/hour. After three days, if adverse events were tolerated, the dose was increased to BUTRANS 10 mcg/hour. If adverse effects were tolerated but adequate analgesia was not reached, the dose was increased to BUTRANS 20 mcg/hour for an additional 10-12 days. Patients who achieved adequate analgesia and tolerable adverse effects on BUTRANS 10 or 20 mcg/hour were then randomized to remain on their titrated dose of BUTRANS or matching placebo. Fifty-three percent of the patients who entered the open-label titration period were able to titrate to a tolerable and effective dose and were randomized into a 12-week, double-blind treatment period. Twenty-three percent of patients discontinued due to an adverse event from the open-label titration period and 14% discontinued due to lack of a therapeutic effect. The remaining 10% of patients were dropped due to various administrative reasons.
During the first seven days of double-blind treatment patients were allowed up to two tablets per day of immediate-release oxycodone 5 mg as supplemental analgesia to minimize opioid withdrawal symptoms in patients randomized to placebo. Thereafter, the supplemental analgesia was limited to either acetaminophen 500 mg or ibuprofen 200 mg at a maximum of four tablets per day. Sixty-six percent of the patients treated with BUTRANS completed the 12-week treatment compared to 70% of the patients treated with placebo. Of the 256 patients randomized to BUTRANS, 9% discontinued due to lack of efficacy and 16% due to adverse events. Of the 283 patients randomized to placebo, 13% discontinued due to lack of efficacy and 7% due to adverse events.
Of the patients who were randomized, the mean pain (SE) NRS scores were 7.2 (0.08) and 7.2 (0.07) at screening and 2.6 (0.08) and 2.6 (0.07) at pre-randomization (beginning of double-blind phase) for the BUTRANS and placebo groups, respectively.
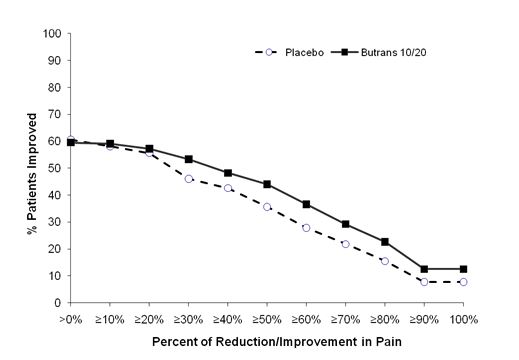
The score for average pain over the last 24 hours at the end of the study (Week 12/Early Termination) was statistically significantly lower for patients treated with BUTRANS compared with patients treated with placebo. The proportion of patients with various degrees of improvement, from screening to study endpoint, is shown in Figure 3 below.
Figure 3. Percent Reduction in Pain Intensity:
12-Week Study in Opioid-Experienced Patients with Chronic Low Back Pain
One thousand one hundred and sixty (1,160) patients on chronic opioid therapy (total daily dose 30-80 mg morphine equivalent) entered an open-label, dose-titration period with BUTRANS for up to 3 weeks, following taper of prior opioids. Patients initiated therapy with BUTRANS 10 mcg/hour for three days. After three days, if the patient tolerated the adverse effects, the dose was increased to BUTRANS 20 mcg/hour for up to 18 days. Patients with adequate analgesia and tolerable adverse effects on BUTRANS 20 mcg/hour were randomized to remain on BUTRANS 20 mcg/hour or were switched to a low-dose control (BUTRANS 5 mcg/hour) or an active control. Fifty-seven percent of the patients who entered the open-label titration period were able to titrate to and tolerate the adverse effects of BUTRANS 20 mcg/hour and were randomized into a 12-week double-blind treatment phase. Twelve percent of patients discontinued due to an adverse event and 21% discontinued due to lack of a therapeutic effect during the open-label titration period.
During the double-blind period, patients were permitted to take ibuprofen (200 mg tablets) or acetaminophen (500 mg tablets) every 4 hours as needed for supplemental analgesia (up to 3200 mg of ibuprofen and 4 grams of acetaminophen daily). Sixty-seven percent of patients treated with BUTRANS 20 mcg/hour and 58% of patients treated with BUTRANS 5 mcg/hour completed the 12-week treatment. Of the 219 patients randomized to BUTRANS 20 mcg/hour, 11% discontinued due to lack of efficacy and 13% due to adverse events. Of the 221 patients randomized to BUTRANS 5 mcg/hour, 24% discontinued due to lack of efficacy and 6% due to adverse events.
Of the patients who were able to be randomized in the double-blind period, the mean pain (SE) NRS scores were 6.4 (0.08) and 6.5 (0.08) at screening and were 2.8 (0.08) and 2.9 (0.08) at pre-randomization (beginning of Double-Blind Period) for the BUTRANS 5 mcg/hour and BUTRANS 20 mcg/hour, respectively.
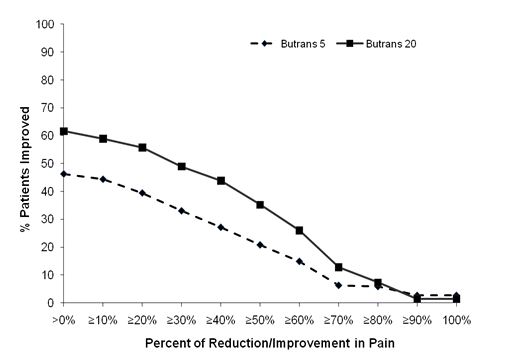
The score for average pain over the last 24 hours at Week 12 was statistically significantly lower for subjects treated with BUTRANS 20 mcg/hour compared to subjects treated with BUTRANS 5 mcg/hour. A higher proportion of BUTRANS 20 mcg/hour patients (49%) had at least a 30% reduction in pain score from screening to study endpoint when compared to BUTRANS 5 mcg/hour patients (33%). The proportion of patients with various degrees of improvement from screening to study endpoint is shown in Figure 4 below.
Figure 4. Percent Reduction in Pain Intensity:
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.