CAMPATH Solution for injection Ref.[50384] Active ingredients: Alemtuzumab
Source: FDA, National Drug Code (US) Revision Year: 2021
12.1. Mechanism of Action
CAMPATH binds to CD52, an antigen present on the surface of B and T lymphocytes, a majority of monocytes, macrophages, NK cells, and a subpopulation of granulocytes. A proportion of bone marrow cells, including some CD34+ cells, express variable levels of CD52. The proposed mechanism of action is antibody-dependent cellular-mediated lysis following cell surface binding of CAMPATH to the leukemic cells.
12.2. Pharmacodynamics
Cardiac Electrophysiology
The effect of multiple doses of alemtuzumab (12 mg/day for 5 days) on the QTc interval was evaluated in a single-arm study in 53 patients without malignancy. No large changes in the mean QTc interval (i.e., >20 ms) were detected in the study. A mean increase in heart rate of 22 to 26 beats/min was observed for at least 2 hours following the initial infusion of alemtuzumab. This increase in heart rate was not observed with subsequent doses.
12.3. Pharmacokinetics
CAMPATH pharmacokinetics were characterized in a study of 30 previously treated B-CLL patients in whom CAMPATH was administered at the recommended dose and schedule. After 12 weeks of dosing, patients exhibited a 7-fold increase in mean AUC.
Distribution
After the last 30 mg dose, the mean volume of distribution at steady-state was 0.18 L/kg (range 0.1 to 0.4 L/kg).
Elimination
CAMPATH pharmacokinetics displayed nonlinear elimination kinetics. Systemic clearance decreased with repeated administration due to decreased receptor-mediated clearance (i.e., loss of CD52 receptors in the periphery). Mean half-life was 11 hours (range 2 to 32 hours) after the first 30 mg dose and was 6 days (range 1 to 14 days) after the last 30 mg dose.
Specific Populations
The effects of renal or hepatic impairment on the pharmacokinetics of CAMPATH have not been studied.
13.1. Carcinogenesis, Mutagenesis, Impairment of Fertility
Studies to assess the carcinogenic or genotoxic potential of CAMPATH have not been conducted.
In fertility studies, alemtuzumab (3 or 10 mg/kg intravenously) was administered to huCD52 transgenic male mice on 5 consecutive days prior to cohabitation with untreated wild-type females. No effect on fertility or reproductive performance was observed. However, adverse effects on sperm parameters (including abnormal morphology [detached/no head] and reduced total count and motility) were observed at both doses tested.
When alemtuzumab (3 or 10 mg/kg intravenously) was administered to huCD52 transgenic female mice for 5 consecutive days prior to cohabitation with untreated wild-type males, there was a decrease in the average number of corpora lutea and implantation sites and an increase in postimplantation loss, resulting in fewer viable embryos at the higher dose tested.
14. Clinical Studies
14.1 Previously Untreated B-CLL Patients
CAMPATH was evaluated in an open-label, randomized (1:1) active-controlled study in previously untreated patients with B-CLL, Rai Stage I–IV, with evidence of progressive disease requiring therapy. Patients received either CAMPATH 30 mg intravenously 3 times per week for a maximum of 12 weeks or chlorambucil 40 mg/m² orally once every 28 days for a maximum of 12 cycles.
Of the 297 patients randomized, the median age was 60 years, 72% were male, 99% were Caucasian, 96% had a WHO performance status 0–1, 23% had maximum lymph node diameter ≥5 cm, 34% were Rai Stage III/IV, and 8% were treated in the U.S.
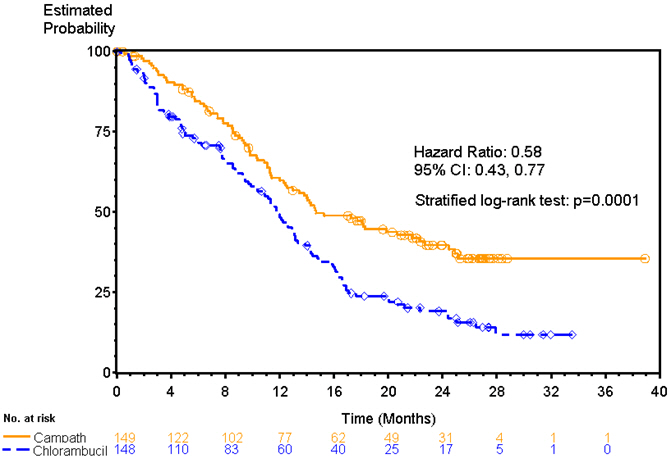
Patients randomized to receive CAMPATH experienced longer progression free survival (PFS) compared to those randomized to receive chlorambucil (median PFS 14.6 months vs. 11.7 months, respectively). The overall response rates were 83% and 55% (p <0.0001) and the complete response rates were 24% and 2% (p<0.0001) for CAMPATH and chlorambucil arms, respectively. The Kaplan-Meier curve for PFS is shown in Figure 1.
Figure 1. Progression Free Survival in Previously Untreated B-CLL Patients*:
14.2 Previously Treated B-CLL Patients
CAMPATH was evaluated in three multicenter, open-label, single-arm studies of 149 patients with B-CLL previously treated with alkylating agents, fludarabine, or other chemotherapies. Patients were treated with the recommended dose of CAMPATH 30 mg intravenously 3 times per week for up to 12 weeks. Partial response rates of 21% to 31% and complete response rates of 0% to 2% were observed.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.