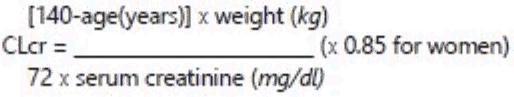CETRIZ Film coated tablet Ref.[49821] Active ingredients: Cetirizine
Source: Health Products Regulatory Authority (IE) Revision Year: 2021 Publisher: Actavis Group PTC ehf, Reykjavikurvegi 76-78, 220 Hafnarfjördur, Iceland
4.1. Therapeutic indications
Cetirizine dihydrochloride 10 mg film-coated tablets are indicated in adults and paediatric patients 6 years and above:
- for the relief of nasal and ocular symptoms of seasonal and perennial allergic rhinitis.
- for the relief of symptoms of chronic idiopathic urticaria.
4.2. Posology and method of administration
Posology
Adults and adolescents over 12 years of age: 10 mg once daily (1 tablet).
Special population
Elderly
Data do not suggest that the dose needs to be reduced in elderly subjects provided that the renal function is normal.
Renal impairment
There are no data to document the efficacy/ safety ratio in patients with renal impairment. Since cetirizine is mainly excreted via renal route (see section 5.2), in case no alternative treatment can be used, the dosing intervals must be individualised according to renal function. Refer to the following table and adjust the dose as indicated. To use this dosing table, an estimate of the patient’s creatinine clearance (CLcr) in ml/min is needed. The CLcr (ml/min) may be estimated from serum creatinine (mg/dl) determination using the following formula:
Dosing adjustments for adult patients with impaired renal function:
| Group | Creatinine clearance (ml/min) | Dosage and frequency |
|---|---|---|
| Normal | ≥80 | 10 mg once daily |
| Mild | 50–79 | 10 mg once daily |
| Moderate | 30–49 | 5 mg once daily |
| Severe | <30 | 5 mg once every 2 days |
| End-stage renal disease | <10 | Contra-indicated |
| Patients undergoing dialysis |
Hepatic impairment
No dose adjustment is needed in patients with solely hepatic impairment. In patients with hepatic impairment and renal impairment, adjustment of the dose is recommended (see Renal impairment above).
Paediatric population
The tablet formulation should not be used in children under 6 years of age as it does not allow the necessary dose adjustments.
Children aged 6 to 12 years: 5 mg twice daily (a half tablet twice daily).
Adolescents above 12 years: 10 mg once daily (1 tablet).
In paediatric patients suffering from renal impairment, the dose will have to be adjusted on an individual basis taking into account the renal clearance, age and body weight of the patient.
Method of administration
The tablets need to be swallowed with a glass of liquid.
4.9. Overdose
Symptoms
Symptoms observed after an important overdose of cetirizine are mainly associated with CNS effects or with effects that could suggest an anticholinergic effect.
Adverse events reported after an intake of at least 5 times the recommended daily dose are: confusion, diarrhoea, dizziness, fatigue, headache, malaise, mydriasis, pruritus, restlessness, sedation, somnolence, stupor, tachycardia, tremor, and urinary retention.
Management
There is no known specific antidote to cetirizine.
Should overdose occur, symptomatic or supportive treatment is recommended. Gastric lavage should be considered shortly after ingestion of the drug.
Cetirizine is not effectively removed by haemodialysis.
6.3. Shelf life
3 years.
6.4. Special precautions for storage
No special precautions.
6.5. Nature and contents of container
Aluminium/Aluminium blisters.
Pack size: 7,10,14, 28 or 30 Tablets.
Not all pack sizes may be marketed.
6.6. Special precautions for disposal and other handling
No special requirements.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.