CEVENFACTA Powder and solvent for solution for injection Ref.[50508] Active ingredients: Coagulation factor VIIa
Source: European Medicines Agency (EU) Revision Year: 2022 Publisher: Laboratoire français du Fractionnement et des Biotechnologies, Tour W, 102 Terrasse Boieldieu, 19ème Étage, 92800 Puteaux, France
4.1. Therapeutic indications
CEVENFACTA is indicated in adults and adolescents (12 years of age and older) for the treatment of bleeding episodes and for the prevention of bleeding in those undergoing surgery or invasive procedures in the following patient groups:
- in patients with congenital haemophilia with high-responding inhibitors to coagulation factors VIII or IX (i.e. ≥5 Bethesda Units (BU));
- in patients with congenital haemophilia with low titre inhibitors (BU <5), but expected to have a high anamnestic response to factor VIII or factor IX administration or expected to be refractory to increased dosing of FVIII or FIX.
4.2. Posology and method of administration
Treatment should be initiated and supervised by a physician experienced in the treatment of haemophilia and/or bleeding disorders.
Posology
The dose and duration of treatment depend on the location and severity of the bleeding or the type of surgery/procedure, the need for urgent haemostasis, the frequency of administration, and the known patient responsiveness to FVIIa-containing bypassing agents during prior bleeding events.
The results of laboratory assessment(s) of coagulation (prothrombin time (PT)/international normalised ratio (INR), activated partial thromboplastin time (aPTT), FVII coagulation activity (Clotting time) (FVII:C)) do not necessarily correlate with or predict the haemostatic effectiveness of this medicinal product.
The dose, frequency, and duration of CEVENFACTA therapy should be based on the patient’s clinical response and haemostasis evaluation.
Maximum tolerated doses have not been determined for this medicinal product and cumulative daily doses greater than 1 025 μg/kg have not been studied.
Treatment of bleeding episodes
Treatment with this medicinal product should be initiated as soon as a bleeding event occurs.
The recommended initial dose should be adjusted based on the criteria provided in Table 1.
For mild to moderate bleeding episodes, the duration of home therapy should not exceed 24 hours. Only after consultation with the haemophilia treatment centre can continued home treatment be considered.
If signs or symptoms of severe bleeding occur in the home setting, immediate medical care should be sought by patients. In the meantime, to avoid any treatment delay, an initial dose can be administered at home.
In all situations, if an adequate haemostatic response is not achieved (e.g., within 24 hours of the first administration of CEVENFACTA for mild and moderate bleeding episodes), alternative therapies should be considered.
Table 1. Dosing for the treatment of bleeding episodes:
| Type of bleeding | Dosing regimen recommendation | Duration of therapy |
|---|---|---|
| Mild and moderate Joint, superficial muscle, soft tissue, and mucous membranes. | 75 μg/kg repeated every 3 hours until haemostasis is achieved. or Initial dose of 225 μg/kg. If haemostasis is not achieved within 9 hours, additional 75 μg/kg doses may be administered every 3 hours as needed to achieve haemostasis. The following factors should be considered when choosing the initial dose of this medicinal product: • The severity and site of bleeding and need for urgent haemostasis • Frequency of administration • Known patient responsiveness to FVIIa- containing bypassing agents during prior bleeding events | Continue therapy to support healing and prevent recurrent haemorrhage after haemostasis to maintain the haemostatic plug. The site and severity of bleeding should determine therapy duration. |
| Severe Life or limb threatening haemorrhage, iliopsoas and deep muscle with neurovascular injury, retroperitoneum, intracranial, or gastrointestinal. | 225 μg/kg initially, followed if necessary 6 hours later with 75 μg/kg every 2 hours until haemostasis is achieved. Subsequent dosing: After achieving haemostasis, the decision for dosing should be based on the clinical assessment and the type of bleeding bearing in mind relevant warning and precautions (see section 4.4). | Continue therapy to support healing and prevent recurrent haemorrhage. The site and severity of bleeding and the use of other procoagulant therapies should determine therapy duration. |
There was limited experience with severe bleedings in the PerSept 1 clinical study.
Prevention of bleeding during surgical or invasive procedures
CEVENFACTA dosing for the prevention of bleeding during surgical or invasive procedures (perioperative management) is provided in Table 2.
Table 2. Dosing for perioperative management of bleeding:
| Type of surgical procedure | Dosing regimen recommendation | Duration of therapy |
|---|---|---|
| Minor Including uncomplicated tooth extraction, peripheral central catheter insertion, Port-a-Cath placement, etc. | Initial dose: 75 μg/kg immediately before surgery or start of invasive procedure; then Subsequent doses: 75 μg/kg repeated every 2 hours for the first 48 hours following the initial dose. | Most minor procedures should be treated for 48 hours to achieve haemostasis. At the discretion of the clinician, this medicinal product may be administered less frequently than every 2 hours and/or for less than 48 hours. |
| Major | Pre-operative and operative doses: 200 μg/kg immediately before the surgery, followed by 75 μg/kg every 2 hours for the duration of the surgery The following post-operative doses may be administered: • First 48 hours: 75 μg/kg every 2 hours • Days 3-4: 75 μg/kg every 2 to 4 hours • Days 5-6: 75 μg/kg every 2 to 6 hours • Days 7-10: 75 μg/kg every 2 to 8 hours • Day 11 onwards: 75 μg/kg every 2 to 12 hours The dose and dosing intervals may be adjusted by the healthcare provider based on the clinical assessment and known patient responsiveness to FVIIa-containing bypassing agents. Following the surgery, CEVENFACTA (75 μg/kg) is also recommended prior to drain or suture removal or physical therapy. | This medicinal product should be administered for a minimum of 5 postoperative days (120 hours) and for as long as necessary to achieve haemostasis and support wound healing. |
Close follow-up is important for early detection of potential postoperative bleeding events that may require adjustment of the dosing intervals.
Special population
The dosing regimen in elderly patients and in patients with renal or hepatic impairment has not yet been established (see sections 4.4 and 5.2).
Paediatric population
The efficacy of CEVENFACTA in children <12 years has not been established. Currently available data are described in sections 4.8 and 5.1 but no recommendation on a posology can be made.
In line with the European Medicines Agency recommendations, there is no relevant use of CEVENFACTA for the treatment of congenital haemophilia in the paediatric population from birth to less than 6 months.
Method of administration
For instructions on reconstitution of the medicinal product before administration, see section 6.6. Administer the solution as an intravenous bolus injection over 2 minutes or less.
4.9. Overdose
There is no experience of overdose in clinical studies.
The dosing schedule should not be intentionally increased above the recommended doses due to the absence of information on the additional risk that may be incurred.
6.3. Shelf life
3 years.
After reconstitution, the product must be stored in the vial and administered within 4 hours. Any unused solution should be discarded 4 hours after reconstitution.
For more information on instructions for reconstitution please refer to section 6.6.
6.4. Special precautions for storage
Store below 30°C.
Do not freeze.
Keep the vial in the outer carton in order to protect from light.
For storage conditions of the reconstituted medicinal product, see section 6.3.
6.5. Nature and contents of container
Each pack contains:
CEVENFACTA 1 mg (45 KIU) powder and solvent for solution for injection:
- 1 glass vial with powder (1 mg) for solution for injection,
- 1 sterile vial adapter for reconstitution equipped with a 5 µm filter,
- 1 prefilled syringe of water for injections (1.1 mL),
- 1 plunger rod and backstop.
CEVENFACTA 2 mg (90 KIU) powder and solvent for solution for injection:
- 1 glass vial with powder (2 mg) for solution for injection,
- 1 sterile vial adapter for reconstitution equipped with a 5 µm filter,
- 1 prefilled syringe of water for injections (2.2 mL),
- 1 plunger rod and backstop.
CEVENFACTA 5 mg (225 KIU) powder and solvent for solution for injection:
- 1 glass vial with powder (5 mg) for solution for injection,
- 1 sterile vial adapter for reconstitution equipped with a 5 µm filter,
- 1 prefilled syringe of water for injections (5.2 mL),
- 1 plunger rod and backstop.
6.6. Special precautions for disposal and other handling
After reconstitution with the supplied set, the solution appears as a clear to slightly turbid colourless liquid free of foreign particles.
The reconstituted medicinal product should be inspected visually for particulate matter prior to administration. Do not use solutions that are cloudy or have deposits.
Instructions for reconstitution
Aseptic technique and a flat work surface should always be used during the reconstitution procedure.
1. CEVENFACTA powder vial and pre-filled syringe with solvent should be at room temperature (between 15°C and 25°C) at reconstitution.
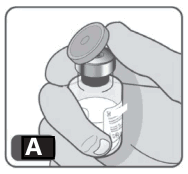
2. Remove the plastic cap from the vial (Fig A). If the cap is lost or missing, do not use the vial.
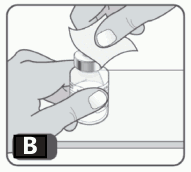
3. Wipe the rubber stopper on the vial with an alcohol swab. Allow the alcohol to dry. After cleaning with the swab, do not touch the rubber stopper with your fingers and don’t allow it to touch any other object until you attach the vial adapter, as this can transfer germs (Fig B).
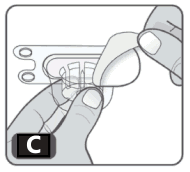
4. Open the vial adapter package by peeling off the protective paper cover, without touching the inside. Do not remove the vial adapter from the package. The spike of the adapter should line up with the middle of the grey rubber stopper (Fig C).
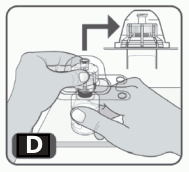
5. Turn the package over. Firmly press down to fully insert the vial adapter spike through the rubber stopper of the vial (Fig D).
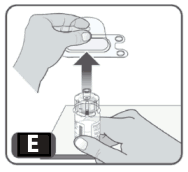
6. Lightly squeeze the plastic cover and lift up to remove it from the vial adapter. Do not touch the exposed spike of the vial adapter (Fig E).
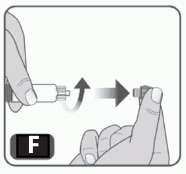
7. Remove the syringe cap from the pre-filled syringe by holding the syringe body with one hand and using the other hand to unscrew the syringe cap (turn to the left). Do not touch the syringe tip. Do not use the prefilled syringe if the syringe cap is lost or missing (Fig F).
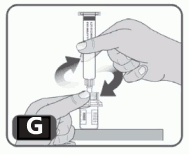
8. While holding the edges of the vial adapter screw on the prefilled syringe (turn to the right) a few turns until it starts to tighten. Be careful not to overtighten as you will need to remove the syringe later (Fig G).
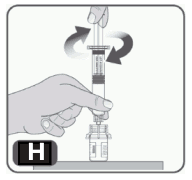
9. Hold the plunger rod by the wide top end in one hand and the syringe body using your other hand. Insert the plunger rod into the syringe, and then screw a few turns (turn to the right) so that the plunger rod is attached to the grey rubber stopper in the syringe (Fig H).
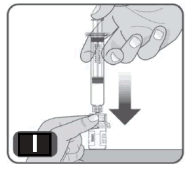
10. Very slowly push the plunger rod down to the bottom of the syringe, in order to transfer all of the liquid from the syringe into the vial. Do not push too quickly as it can result in excess foam and air in the vial (Fig I).
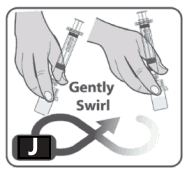
11. Swirl the vial gently or roll between hands until all powder is dissolved. Do not shake the vial as this creates foam and air (Fig J).
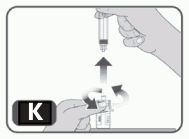
12. Without withdrawing any medicinal product back into the syringe, unscrew the syringe from the vial adapter (turn to the left) until it is completely detached. Don’t remove the vial adapter from the vial (Fig K).
13. Withdraw the liquid medicinal product from the vial(s), using a syringe provided by your specialty pharmacy that is large enough to hold your prescribed dose.
If your dose requires more than one vial, repeat the above steps with additional kits until you have reached your required dose.
Instructions for administration
The medicinal product must be administered within 4 hours of reconstitution.
The medicinal product can be administered in 2 minutes or less as an intravenous infusion.
Instructions for disposal
Any unused medicinal product or waste material should be disposed of in accordance with local requirements.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.