CITANEST PLAIN Solution for injection Ref.[27506] Active ingredients: Prilocaine
Source: FDA, National Drug Code (US) Revision Year: 2019
Product description
4% Citanest Plain Dental (prilocaine HCl Injection, USP), is a sterile, non pyrogenic isotonic solution that contains a local anesthetic agent and is administered parenterally by injection. See INDICATIONS AND USAGE for specific uses. The quantitative composition is shown in Table 1.
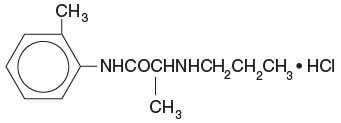
4% Citanest Plain Dental contains prilocaine HCl, which is chemically designated as propanamide, N-(2-methyl-phenyl)2(propylamino)-, monohydrochloride and has the following structural formula:
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration.
The specific quantitative composition is shown in Table 1.
Table 1. Composition:
| Product Identification | Formula (mg/mL) | |
|---|---|---|
| Prilocaine HCl | pH | |
| 4% Citanest Plain Dental | 40.0 | 6.0 to 7.0 |
Note: Sodium hydroxide or hydrochloric acid may be used to adjust the pH of 4% Citanest Plain Dental Injection.
| How Supplied |
|---|
|
4% Citanest Plain Dental Injection (NDC 66312-630-14) is dispensed in 1.8 mL cartridges, packed 50 per box. Manufactured for Dentsply Pharmaceutical, York, PA 17404 |
Drugs
| Drug | Countries | |
|---|---|---|
| CITANEST | Malta, Netherlands, New Zealand, Turkey, United Kingdom, United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.