CLOROTEKAL Solution for injection Ref.[28062] Active ingredients: Chloroprocaine
Source: FDA, National Drug Code (US) Revision Year: 2020
Product description
CLOROTEKAL is a sterile non pyrogenic local anesthetic.
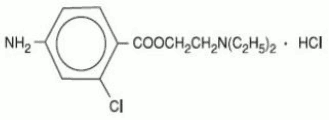
The active ingredient in CLOROTEKAL is chloroprocaine hydrochloride (benzoic acid, 4-amino-2-chloro-2-(diethylamino) ethyl ester, monohydrochloride), an ester local anesthetic, which is represented by the following structural formula:
1 mL of solution for injection contains 10 mg of chloroprocaine hydrochloride, equivalent to 44.05 mg/5 mL (8.81 mg/mL) chloroprocaine. It also contains the following inactive ingredients: hydrochloric acid 1N (for pH adjustment), sodium chloride, water for injection. The pH of the solution is between 3.0 and 4.0. The osmolality of the solution is 270-300 mOsm/kg.
| Dosage Forms and Strengths |
|---|
|
CLOROTEKAL is supplied as a single-dose sterile, clear, colorless solution in a Type I (USP) glass ampule that provides 50 mg of chloroprocaine hydrochloride in 5 mL aqueous solution (concentration: 10 mg/mL) equivalent to 44.05 mg/5 mL (8.81 mg/mL) chloroprocaine. |
| How Supplied | ||||||
|---|---|---|---|---|---|---|
|
The CLOROTEKAL is supplied as a 50mg/5mL (10 mg/mL) equivalent to 44.05 mg/5 mL (8.81 mg/mL) chloroprocaine Type I glass ampules, stored in cartons containing 10 single-dose ampules.
The product is intended for intrathecal administration. Solutions which are discolored or which contain particulate matter should not be administered. Handling
Manufactured by: Sintetica S.A., Switzerland Manufactured for: B. Braun Medical Inc., Bethlehem, PA 18018-3524, USA |
Drugs
| Drug | Countries | |
|---|---|---|
| CLOROTEKAL | Estonia, France, Croatia, United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.