CO-DIOVAN Film-coated tablet Ref.[50869] Active ingredients: Hydrochlorothiazide Valsartan
Source: Pharmaceutical Benefits Scheme (AU) Revision Year: 2022 Publisher: Novartis Pharmaceuticals Australia Pty Limited, ABN 18 004 244 160, 54 Waterloo Road, Macquarie Park NSW 2113
Product name and form
Co-Diovan 80/12.5 (valsartan/hydrochlorothiazide) film-coated tablet.
Co-Diovan 160/12.5 (valsartan/hydrochlorothiazide) film-coated tablet.
Co-Diovan 160/25 (valsartan/hydrochlorothiazide) film-coated tablet.
Co-Diovan 320/12.5 (valsartan/hydrochlorothiazide) film-coated tablet.
Co-Diovan 320/25 (valsartan/hydrochlorothiazide) film-coated tablet.
| Pharmaceutical Form |
|---|
|
Co-Diovan 80/12.5: Ovaloid, non-divisible, film-coated tablets coloured light orange and imprinted with HGH on one side and CG on the other side. Co-Diovan 160/12.5: Ovaloid, non-divisible, film-coated tablets coloured dark red and imprinted with HHH on one side and CG on the other side. Co-Diovan 160/25: Ovaloid, non-divisible, film-coated tablets coloured brown-orange and imprinted with HXH on one side and NVR on the other side. Co-Diovan 320/12.5: Ovaloid, non-divisible, film-coated tablets coloured pink and imprinted with HIL on one side and NVR on the other side. Co-Diovan 320/25: Ovaloid, non-divisible, film-coated tablets coloured yellow and imprinted with CTI on one side and NVR on the other side. Co-Diovan tablets cannot be divided into equal doses. |
Qualitative and quantitative composition
Valsartan [N-Pentanoyl-N-[2'-(1H-tetrazol-5-yl)biphenyl-4ylmethyl]-L-valine] is a fine white powder soluble in methanol, neutral and basic aqueous solutions, and very slightly soluble in solutions with pH <7.
Hydrochlorothiazide [6-chloro-3,4-dihydro-2H-1,2,4-benzothiadiazine-7-sulfonamide-1,1-dioxide] is structurally related to the thiazide group of diuretics. It is a white or almost white powder. Hydrochlorothiazide is very slightly soluble in water and freely soluble in dimethylsulfoxide.
Co-Diovan 80/12.5 contains valsartan 80 mg and hydrochlorothiazide 12.5 mg; Co-Diovan 160/12.5 contains valsartan 160 mg and hydrochlorothiazide 12.5 mg; Co-Diovan 160/25 contains valsartan 160 mg and hydrochlorothiazide 25 mg; Co-Diovan 320/12.5 contains valsartan 320 mg and hydrochlorothiazide 12.5 mg; Co-Diovan 320/25 contains valsartan 320 mg and hydrochlorothiazide 25 mg.
Co-Diovan is available in five strengths: Co-Diovan 80/12.5, Co-Diovan 160/12.5, Co-Diovan 160/25, Co-Diovan 320/12.5 and Co-Diovan 320/25.
For the full list of excipients, see Section 6.1 List of excipients.
Chemical structure:
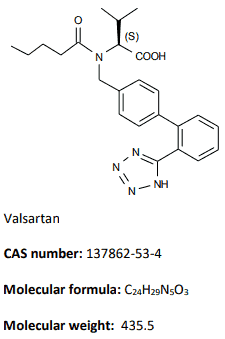 | 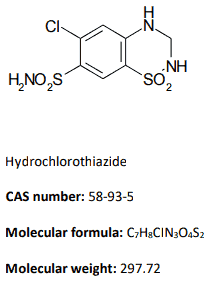 |
| Active Ingredient |
|---|
|
Hydrochlorothiazide is a diuretic with antihypertensive properties. It acts by inhibiting the renal tubular re-absorption of sodium and chloride ions, which are excreted with an accompanying volume of water. Potassium excretion is also promoted. |
|
Valsartan is an orally active, potent, and specific angiotensin II (Ang II) receptor antagonist. It acts selectively on the AT1-receptor subtype, which is responsible for the known actions of angiotensin II. |
| List of Excipients |
|---|
|
Co-Diovan 80/12.5: silica-colloidal anhydrous; crospovidone; hypromellose; magnesium stearate; cellulose-microcrystalline; macrogol 8000; talc-purified; titanium dioxide; iron oxide red; iron oxide yellow. Co-Diovan 160/12.5: silica-colloidal anhydrous; crospovidone; hypromellose; magnesium stearate; cellulose-microcrystalline; macrogol 8000; talc-purified; titanium dioxide; iron oxide red. Co-Diovan 160/25: silica-colloidal anhydrous; crospovidone; hypromellose; magnesium stearate; cellulose-microcrystalline; macrogol 4000; talc-purified; titanium dioxide, iron oxide red, iron oxide yellow, iron oxide black. Co-Diovan 320/12.5: silica-colloidal anhydrous; crospovidone; hypromellose; magnesium stearate; cellulose-microcrystalline; macrogol 4000; talc-purified; titanium dioxide, iron oxide red, iron oxide black. Co-Diovan 320/25: silica-colloidal anhydrous; crospovidone; hypromellose; magnesium stearate; cellulose-microcrystalline; macrogol 4000; talc-purified; titanium dioxide, iron oxide yellow. |
Pack sizes and marketing
Co-Diovan 80/12.5: PA/Al/PVC/Al blister packs of 7, 14, 28, 30 and 56.
Co-Diovan 160/12.5: PA/Al/PVC/Al blister packs of 7, 14, 28, 30 and 56.
Co-Diovan 160/25: PA/Al/PVC/Al blister packs of 7, 14, 28, 30 and 56.
Co-Diovan 320/12.5: PA/Al/PVC/Al blister packs of 7, 14, 28, 30 and 56.
Co-Diovan 320/25: PA/Al/PVC/Al blister packs of 7, 14, 28, 30 and 56.
Not all strengths and pack sizes may be marketed.
Marketing authorization holder
Novartis Pharmaceuticals Australia Pty Limited, ABN 18 004 244 160, 54 Waterloo Road, Macquarie Park NSW 2113
Marketing authorization dates and numbers
31 May 2005
Drugs
| Drug | Countries | |
|---|---|---|
| CO-DIOVAN | Austria, Australia, Cyprus, Germany, Estonia, Spain, Hong Kong, Croatia, Ireland, Israel, Lithuania, Malta, Mexico, Nigeria, Netherlands, New Zealand, Poland, Singapore, Turkey, United Kingdom, South Africa |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.