COLY-MYCIN M Solution for injection Ref.[50379] Active ingredients: Colistimethate
Source: FDA, National Drug Code (US) Revision Year: 2018
Product description
Coly-Mycin M Parenteral (Colistimethate for Injection, USP) is a sterile parenteral antibiotic product which, when reconstituted (see Reconstitution), is suitable for intramuscular or intravenous administration.
Each vial contains colistimethate sodium or pentasodium colistinmethanesulfonate (150 mg colistin base activity).
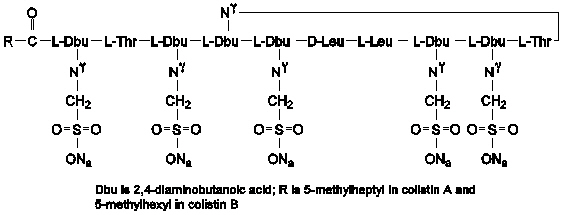
Colistimethate sodium is a polypeptide antibiotic with an approximate molecular weight of 1750. The empirical formula is C58H105N16Na5O28S5 and the structural formula is represented below:
| How Supplied |
|---|
|
Coly-Mycin M Parenteral is supplied in vials containing colistimethate sodium (equivalent to 150 mg colistin base activity per vial) as a white to slightly yellow lyophilized cake. NDC 42023-107-01: one individual vial. Distributed by: Par Pharmaceutical, Chestnut Ridge, NY 10977 |
Drugs
| Drug | Countries | |
|---|---|---|
| COLY-MYCIN M | New Zealand, United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.