COTELLIC Film-coated tablet Ref.[7186] Active ingredients: Cobimetinib
Source: European Medicines Agency (EU) Revision Year: 2019 Publisher: Roche Registration GmbH, Emil-Barell-Strasse 1, 79639 Grenzach-Wyhlen, Germany
Pharmacodynamic properties
Pharmacotherapeutic group: Antineoplastic agents
ATC code: L01XE38
Mechanism of action
Cobimetinib is a reversible, selective, allosteric, oral inhibitor that blocks the mitogen-activated protein kinase (MAPK) pathway by targeting the mitogen-activated extracellular signal-regulated kinase (MEK) 1 and MEK 2 which results in inhibition of phosphorylation of the extracellular signalregulated kinase (ERK) 1 and ERK 2. Therefore, cobimetinib blocks the cell proliferation induced by the MAPK pathway through inhibition of the MEK1/2 signalling node.
In the preclinical models, the combination of cobimetinib and vemurafenib showed that by simultaneously targeting mutated BRAF V600 proteins and MEK proteins in melanoma cells, the combination of the two products inhibits MAPK pathway reactivation through MEK1/2, resulting in a stronger inhibition of intracellular signalling and decreased tumour cell proliferation
Clinical efficacy and safety
There are no data on the safety or efficacy of Cotellic in combination with vemurafenib in patients with central nervous system metastasis or in patients with non-cutaneous malignant melanoma.
Study GO28141 (coBRIM)
Study GO28141 is a multi-centre, randomised, double-blind, placebo-controlled, Phase III study to evaluate the safety and efficacy of Cotellic in combination with vemurafenib as compared to vemurafenib plus placebo, in previously untreated patients with BRAF V600 mutation-positive unresectable locally advanced (Stage IIIc) or metastatic melanoma (Stage IV).
Only patients with ECOG performance status 0 and 1 were enrolled in Study GO28141. Patients with ECOG performance status 2 or higher were excluded from the study.
Following confirmation of a BRAF V600 mutation, using the cobas 4800 BRAF V600 mutation test, 495 previously untreated patients with unresectable locally advanced or metastatic melanoma were randomised to receive either:
- Placebo once daily on Days 1-21 of each 28-day treatment cycle and 960 mg vemurafenib twice daily on Days 1-28, or
- Cotellic 60 mg once daily on Days 1-21 of each 28-day treatment cycle and 960 mg vemurafenib twice daily on Days 1-28
Progression-free survival (PFS) as assessed by the investigator (INV) was the primary endpoint. Secondary efficacy endpoints included overall survival (OS), objective response rate, duration of response (DoR) as assessed by INV and PFS as assessed by an independent review facility (IRF).
Key baseline characteristics included: 58% of patients were male, median age was 55 years (range 23 to 88 years), 60% had metastatic melanoma stage M1c and the proportion of patients with elevated LDH was 46.3% in the cobimetinib plus vemurafenib arm and 43.0% in the placebo plus vemurafenib arm.
In Study GO28141, there were 89 patients (18.1%) aged 65-74, 38 patients (7.7%) aged 75-84 and 5 patients (1.0%) aged 85 years and older.
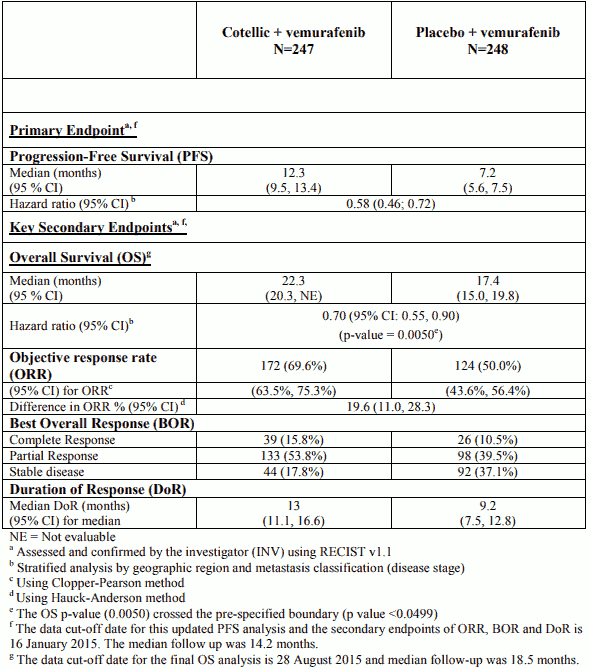
Efficacy results are summarized in Table 5.
Table 5. Efficacy results from Study GO28141 (coBRIM):
The primary analysis for Study GO28141 was conducted with a data cut-off date of 09 May 2014. Significant improvement in the primary endpoint, investigator-assessed PFS, was observed in patients assigned to the Cotellic plus vemurafenib arm compared to the placebo plus vemurafenib arm (HR 0.51 (0.39; 0.68); p-value < 0.0001). The median estimate for investigator-assessed PFS was 9.9 months for the Cotellic plus vemurafenib arm vs. 6.2 months for the placebo plus vemurafenib arm. The median estimate for independent review of PFS was 11.3 months for the Cotellic plus vemurafenib arm vs. 6.0 months for the placebo plus vemurafenib arm (HR 0.60 (0.45; 0.79); p-value = 0.0003). The objective response rate (ORR) in the Cotellic plus vemurafenib arm was 67.6% vs 44.8% in the placebo plus vemurafenib arm. The difference in ORR was 22.9% (p-value<0.0001).
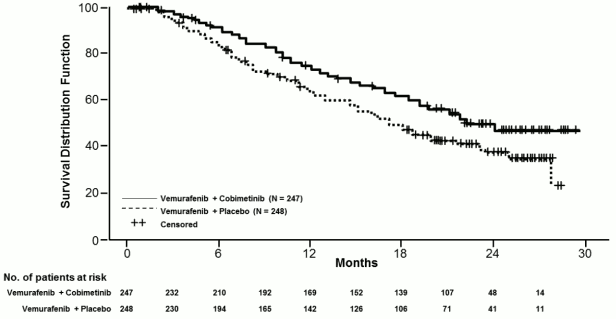
The final OS analysis for Study GO28141 was conducted with a data-cut off date of 28 August 2015. Significant improvement in OS was observed in patients assigned to the Cotellic plus vemurafenib arm compared to the placebo plus vemurafenib arm (Figure 1). The 1-year (75%) and 2-year (48%) OS estimates for the Cotellic plus vemurafenib arm were greater than those for placebo plus vemurafenib arm (64% and 38% respectively).
Figure 1. Kaplan-Meier curves of final overall survival – Intent to treat population (cut-off date: 28 August 2015):
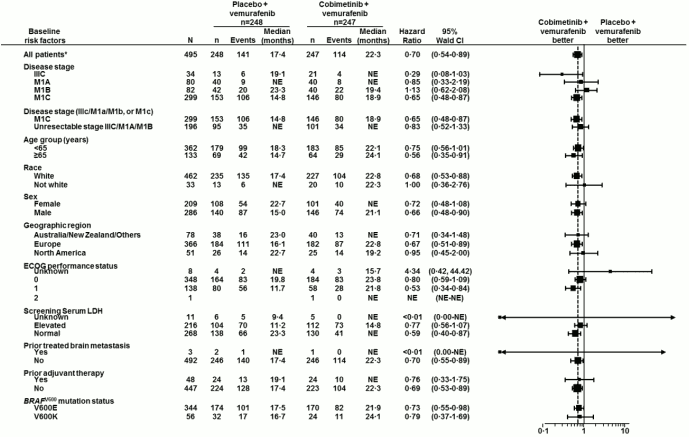
Figure 2. Forest plot for hazard ratios of final overall survival subgroup analyses – Intent to treat population (cut-off date: 28 August 2015):
Global health status / health-related quality of life by patient-report were measured using the EORTC Quality of Life Questionnaire – Core 30 (QLQ-C30). Scores for all functioning domains and most symptoms (appetite loss, constipation, nausea and vomiting, dyspnoea, pain, fatigue) showed that the mean change from baseline was similar between the two treatment arms and did not demonstrate a clinically meaningful change (all scores were ≤ 10 point change from baseline).
Study NO25395 (BRIM7)
The efficacy of Cotellic was evaluated in Phase Ib Study, NO25395, which was designed to assess the safety, tolerability, pharmacokinetics and efficacy of Cotellic when added to vemurafenib for the treatment of patients with BRAFV600 mutation-positive (as detected by the cobas 4800 BRAF V600 Mutation Test), unresectable or metastatic melanoma. This study treated 129 patients with Cotellic and vemurafenib: 63 were BRAF inhibitor (BRAFi) therapy naïve and 66 patients had previously progressed on prior vemurafenib therapy. Among the 63 BRAFi naïve patients, 20 patients had received prior systemic therapy for advanced melanoma with the majority (80%) being immunotherapy.
Results of the BRAFi naïve population from Study NO25395 were generally consistent with those from Study GO28141. The BRAFi-naïve patients (n=63) attained an 87% objective response rate, including a complete response in 16% of patients. The median duration of response was 14.3 months. The median PFS for BRAFi-naïve patients was 13.8 months, with median follow-up time of 20.6 months.
Among patients who had progressed on vemurafenib (n=66), the objective response rate was 15%. The median duration of response was 6.8 months. The median PFS for patients who had progressed on vemurafenib was 2.8 months, with median follow-up time of 8.1 months.
In patients who were naive to BRAF inhibitor therapy, the median overall survival was 28.5 months (95% CI 23.3-34.6). In patients who had progressed on BRAF inhibitor therapy, the median overall survival was 8.4 months (95% CI 6.7-11.1).
Paediatric population
The European Medicines Agency has deferred the obligation to submit the results of studies with Cotellic in one or more subsets of the paediatric population in malignant solid tumours (see section 4.2 for information on paediatric use).
Pharmacokinetic properties
Absorption
Following oral dosing of 60 mg in cancer patients, cobimetinib showed a moderate rate of absorption with a median Tmax of 2.4 hours. The mean steady-state Cmax and AUC0-24 were 273 ng/mL and 4340 ng.h/mL respectively. The mean accumulation ratio at steady state was approximately 2.4-fold. Cobimetinib has linear pharmacokinetics in the dose range of ~3.5 mg to 100 mg.
The absolute bioavailability of cobimetinib was 45.9% (90% CI: 39.7%, 53.1%) in healthy subjects. A human mass balance study was conducted in healthy subjects, and showed that cobimetinib was extensively metabolised and eliminated in faeces. The fraction absorbed was ~88% indicating high absorption and first pass metabolism.
The pharmacokinetics of cobimetinib are not altered when administered in the fed state (high-fat meal) compared with the fasted state in healthy subjects. Since food does not alter the pharmacokinetics of cobimetinib, it can be administered with or without food.
Distribution
Cobimetinib is 94.8% bound to human plasma proteins in vitro. No preferential binding to human red blood cells was observed (blood to plasma ratio 0.93).
The volume of distribution was 1050 L in healthy subjects given an intravenous dose of 2 mg. The apparent volume of distribution was 806 L in cancer patients based on population pharmacokinetic analysis.
Cobimetinib is a substrate of P-gp in vitro. The transport across the blood brain barrier is unknown.
Biotransformation
Oxidation by CYP3A and glucuronidation by UGT2B7 appear to be the major pathways of cobimetinib metabolism. Cobimetinib is the predominant moiety in plasma. No oxidative metabolites greater than 10% of total circulating radioactivity or human specific metabolites were observed in plasma. Unchanged medicinal product in faeces and urine accounted for 6.6% and 1.6% of the administered dose, respectively, indicating that cobimetinib is primarily metabolised with minimal renal elimination. In vitro data indicate cobimetinib is not an inhibitor of OAT1, OAT3 or OCT2.
Elimination
Cobimetinib and its metabolites were characterised in a mass balance study in healthy subjects. On average, 94% of the dose was recovered within 17 days. Cobimetinib was extensively metabolised and eliminated in faeces.
Following intravenous administration of a 2 mg dose of cobimetinib, the mean plasma clearance (CL) was 10.7 L/hr. The mean apparent CL following oral dosing of 60 mg in cancer patients was 13.8 L/hr.
The mean elimination half-life following oral dosing of cobimetinib was 43.6 hours (range: 23.1 to 69.6 hours). Therefore, it may take up to 2 weeks following treatment cessation for cobimetinib to be completely removed from systemic circulation.
Special populations
Based on a population pharmacokinetic analysis, gender, race, ethnicity, baseline ECOG, mild and moderate renal impairment did not affect the pharmacokinetic of cobimetinib. Baseline age and baseline body weight were identified as statistically significant covariates on cobimetinib clearance and volume of distribution respectively. However, sensitivity analysis suggests neither of these covariates had clinically significant impact on steady state exposure.
Gender
Gender does not have an effect on the exposure of cobimetinib, based on a population pharmacokinetic analysis including 210 women and 277 men.
Elderly
Age does not have an effect on the exposure of cobimetinib, based on a population pharmacokinetic analysis including 133 patients ≥65 years of age.
Renal impairment
Based on preclinical data and the human mass balance study, cobimetinib is mainly metabolised, with minimal renal elimination. No formal pharmacokinetic study has been conducted in patients with renal impairment.
A population pharmacokinetic analysis using data from 151 patients with mild renal impairment (creatinine clearance (CRCL) 60 to less than 90 mL/min), 48 patients with moderate renal impairment (CRCL 30 to less than 60 mL/min), and 286 patients with normal renal function (CRCL greater than or equal to 90 mL/min), showed that CRCL had no meaningful influence on exposure of cobimetinib. Mild to moderate renal impairment does not influence cobimetinib exposure based on the population pharmacokinetic analysis. There are minimal data for Cotellic in patients with severe renal impairment.
Hepatic impairment
The pharmacokinetics of cobimetinib were evaluated in 6 subjects with mild hepatic impairment (Child Pugh A), 6 subjects with moderate hepatic impairment (Child Pugh B), 6 subjects with severe hepatic impairment (Child Pugh C) and 10 healthy subjects. Systemic total cobimetinib exposures after a single dose were similar in subjects with mild or moderate hepatic impairment compared to healthy subjects, while subjects with severe hepatic impairment had lower total cobimetinib exposures (AUC0-∞ geometric mean ratio of 0.69 compared to healthy subjects) which is not considered to be clinically significant. Unbound cobimetinib exposures were similar between subjects with mild and moderate hepatic impairment compared to subjects with normal hepatic function while subjects with severe hepatic impairment had approximately 2-fold higher exposures (see section 4.2).
Paediatric population
No studies have been conducted to investigate the pharmacokinetics of cobimetinib in paediatric patients.
Preclinical safety data
Carcinogenicity studies have not been conducted with cobimetinib. Standard genotoxicity studies with cobimetinib were negative.
No dedicated fertility studies in animals have been performed with cobimetinib. In toxicology studies, degenerative changes were observed in reproductive tissues including increased apoptosis/necrosis of corpora lutea and seminal vesicle, epididymal and vaginal epithelial cells in rats, and epididymal epithelial cells in dogs. The clinical relevance of this is unknown.
When administered to pregnant rats, cobimetinib caused embryolethality and foetal malformations of the great vessels and skull at systemic exposures similar to human exposure at recommended dose.
Cardiovascular safety of cobimetinib in combination with vemurafenib has not been evaluated in vivo. In vitro, cobimetinib produced moderate hERG ion channel inhibition (IC50=0.5 µM [266 ng/mL]), which is approximately 18 fold higher than peak plasma concentrations (Cmax) at the 60 mg to be marketed dose (unbound Cmax=14 ng/mL [0.03 µM]).
Toxicity studies in rats and dogs identified generally reversible degenerative changes in the bone marrow, gastrointestinal tract, skin, thymus, adrenal gland, liver, spleen, lymph node, kidney, heart, ovary, and vagina at plasma exposures below clinical efficacious levels. Dose limiting toxicities included skin ulcerations, surface exudates, and acanthosis in the rat and chronic active inflammation and degeneration of the oesophagus associated with varying degrees of gastroenteropathy in dogs.
In a repeat dose toxicity study in juvenile rats, cobimetinib systemic exposures were 2 to11 fold higher on post natal day 10 than on post natal day 38 when exposures were similar to those in adult rats. In juvenile rats, cobimetinib administration resulted in similar changes as seen in the pivotal toxicity studies in adults, including reversible degenerative changes in the thymus and liver, decreased spleen and thyroid/parathyroid weights, increased phosphorus, bilirubin and red blood cell mass and decreased triglycerides. Mortality occurred in juvenile animals at a dose (3 mg/kg) which did not lead to mortalities in adult animals.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.