COTELLIC Film-coated tablet Ref.[7186] Active ingredients: Cobimetinib
Source: European Medicines Agency (EU) Revision Year: 2019 Publisher: Roche Registration GmbH, Emil-Barell-Strasse 1, 79639 Grenzach-Wyhlen, Germany
Therapeutic indications
Cotellic is indicated for use in combination with vemurafenib for the treatment of adult patients with unresectable or metastatic melanoma with a BRAF V600 mutation (see sections 4.4 and 5.1).
Posology and method of administration
Treatment with Cotellic in combination with vemurafenib should only be initiated and supervised by a qualified physician experienced in the use of anticancer medicinal products.
Before starting this treatment, patients must have BRAF V600 mutation-positive melanoma tumour status confirmed by a validated test (see sections 4.4 and 5.1).
Posology
The recommended dose of Cotellic is 60 mg (3 tablets of 20 mg) once daily.
Cotellic is taken on a 28 day cycle. Each dose consists of three 20 mg tablets (60 mg) and should be taken once daily for 21 consecutive days (Days 1 to 21-treatment period); followed by a 7-day break (Days 22 to 28-treatment break). Each subsequent Cotellic treatment cycle should start after the 7-day treatment break has elapsed.
For information on the posology of vemurafenib, please refer to its SmPC.
Duration of treatment
Treatment with Cotellic should continue until the patient no longer derives benefit or until the development of unacceptable toxicity (see Table 1 below).
Missed doses
If a dose is missed, it can be taken up to 12 hours prior to the next dose to maintain the once-daily regimen.
Vomiting
In case of vomiting after administration of Cotellic, the patient should not take an additional dose on that day and treatment should be continued as prescribed the following day.
General dose modifications
The decision on whether to reduce the dose for either or both treatments should be based on the prescriber’s assessment of individual patient safety or tolerability. Dose modification of Cotellic is independent of vemurafenib dose modification.
If doses are omitted for toxicity, these doses should not be replaced. Once the dose has been reduced, it should not be increased at a later time.
Table 1 below gives general Cotellic dose modification guidance.
Table 1. Recommended Cotellic dose modifications:
| Grade (CTC-AE)* | Recommended Cotellic dose |
|---|---|
| Grade 1 or Grade 2 (tolerable) | No dose reduction. Maintain Cotellic at a dose of 60 mg once daily (3 tablets) |
| Grade 2 (intolerable) or Grade 3/4 | |
| 1st Appearance | Interrupt treatment until Grade ≤ 1, restart treatment at 40 mg once daily (2 tablets) |
| 2nd Appearance | Interrupt treatment until Grade ≤ 1, restart treatment at 20 mg once daily (1 tablet) |
| 3rd Appearance | Consider permanent discontinuation |
* The intensity of clinical adverse events graded by the Common Terminology Criteria for Adverse Events v4.0 (CTC-AE)
Dose modification advice for haemorrhage
Grade 4 events or cerebral haemorrhage: Cotellic treatment should be interrupted. Cotellic treatment should be permanently discontinued for haemorrhage events attributed to Cotellic.
Grade 3 events: Cotellic treatment should be interrupted during evaluation to avoid any potential contribution to the event. There is no data on the effectiveness of Cotellic dose modification for haemorrhage events. Clinical judgment should be applied when considering restarting Cotellic treatment. Vemurafenib dosing can be continued when Cotellic treatment is interrupted, if clinically indicated.
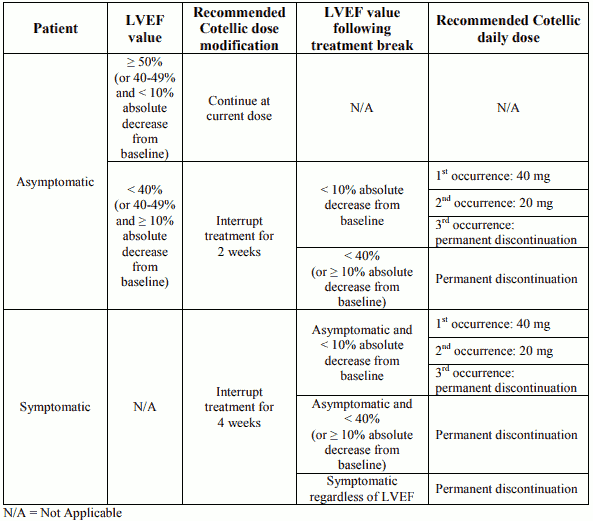
Dose modification advice for left ventricular dysfunction
Permanent discontinuation of Cotellic treatment should be considered if cardiac symptoms are attributed to Cotellic and do not improve after temporary interruption.
Table 2. Recommended dose modifications for Cotellic in patients with left ventricular ejection fraction (LVEF) decrease from baseline:
Vemurafenib treatment can be continued when Cotellic treatment is modified, if clinically indicated.
Dose modification advice for rhabdomyolysis and Creatine phosphokinase (CPK) elevations
Rhabdomyolysis or symptomatic CPK elevations
Cotellic treatment should be interrupted. If rhabdomyolysis or symptomatic CPK elevations do not improve within 4 weeks, Cotellic treatment should be permanently discontinued. If severity is improved by at least one grade within 4 weeks, Cotellic could be restarted at a dose reduced by 20 mg, if clinically indicated. Patients should be closely monitored. Vemurafenib dosing can be continued when Cotellic treatment is modified.
Asymptomatic CPK elevations
Grade 4: Cotellic treatment should be interrupted. If CPK elevations do not improve to Grade ≤3 within 4 weeks following dose interruption, Cotellic treatment should be permanently discontinued.
If CPK improves to Grade ≤3 within 4 weeks, Cotellic could be restarted, if clinically indicated, at a dose reduced by 20 mg and the patient should be closely monitored. Vemurafenib dosing can be continued when Cotellic treatment is modified.
Grade ≤3: After rhabdomyolysis has been ruled out, Cotellic dosing does not need to be modified.
Dose modification advice for Cotellic when used with vemurafenib
Liver laboratory abnormalities
For Grade 1 and 2 liver laboratory abnormalities, Cotellic and vemurafenib should be continued at the prescribed dose.
Grade 3: Cotellic should be continued at the prescribed dose. The dose of vemurafenib may be reduced as clinically appropriate. Please refer to the vemurafenib SmPC.
Grade 4: Cotellic treatment and vemurafenib treatment should be interrupted. If liver laboratory abnormalities improve to Grade ≤1 within 4 weeks, Cotellic should be restarted at a dose reduced by 20 mg and vemurafenib at a clinically appropriate dose, per its SmPC.
Cotellic treatment and vemurafenib treatment should be discontinued if liver laboratory abnormalities do not resolve to Grade ≤1 within 4 weeks or if Grade 4 liver laboratory abnormalities recur after initial improvement.
Photosensitivity
Grade ≤2 (tolerable) photosensitivity should be managed with supportive care.
Grade 2 (intolerable) or Grade ≥3 photosensitivity: Cotellic and vemurafenib should be interrupted until resolution to Grade ≤1. Treatment can be restarted with no change in Cotellic dose. Vemurafenib dosing should be reduced as clinically appropriate, please refer to its SmPC for further information.
Rash
Rash events may occur with either Cotellic or vemurafenib treatment. The dose of Cotellic and/or vemurafenib may be either temporarily interrupted and/or reduced as clinically indicated.
Additionally, for:
Grade ≤2 (tolerable) rash should be managed with supportive care. Cotellic dosing can be continued without modification.
Grade 2 (intolerable) or Grade ≥3 acneiform rash: General dose modification recommendations in Table 1 for Cotellic should be followed. Vemurafenib dosing can be continued when Cotellic treatment is modified (if clinically indicated).
Grade 2 (intolerable) or Grade ≥3 non-acneiform or maculopapular rash: Cotellic dosing can be continued without modification if clinically indicated. Vemurafenib dosing may be either temporarily interrupted and/or reduced, please refer to its SmPC for further information.
QT prolongation
If during treatment the QTc exceeds 500 msec, please refer to the vemurafenib SmPC (section 4.2) for dose modifications for vemurafenib. No dose modification of Cotellic is required when taken in combination with vemurafenib.
Special populations
Elderly patients
No dose adjustment is required in patients aged ≥65 years old.
Renal impairment
No dose adjustment is recommended in patients with mild or moderate renal impairment based on population pharmacokinetic analysis (see section 5.2). There are minimal data for Cotellic in patients with severe renal impairment, therefore an effect cannot be excluded. Cotellic should be used with caution in patients with severe renal impairment.
Hepatic impairment
No dose adjustment is recommended in patients with hepatic impairment. Patients with severe hepatic impairment may have increased plasma concentrations of unbound cobimetinib compared to patients with normal hepatic function (see section 5.2). Liver laboratory abnormalities can occur with Cotellic and caution should be used in patients with any degree of hepatic impairment (see section 4.4).
Non-Caucasian patients
The safety and efficacy of Cotellic in non-Caucasian patients have not been established.
Paediatric population
The safety and efficacy of Cotellic in children and adolescents below 18 years of age have not been established. No data are available.
Method of administration
Cotellic is for oral use. The tablets should be swallowed whole with water. They can be taken with or without food.
Overdose
There is no experience with overdose in human clinical trials. In case of suspected overdose, cobimetinib should be withheld and supportive care instituted. There is no specific antidote for overdosage with cobimetinib.
Shelf life
5 years.
Special precautions for storage
This medicinal product does not require any special storage conditions.
Nature and contents of container
Transparent PVC/PVDC blisters containing 21 tablets.
Each pack contains 63 tablets.
Special precautions for disposal and other handling
Any unused medicinal product or waste material should be disposed of in accordance with local requirements.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.