CYTOVENE-IV Solution for injection Ref.[10800] Active ingredients: Ganciclovir
Source: FDA, National Drug Code (US) Revision Year: 2020
12.1. Mechanism of Action
Ganciclovir is an antiviral drug with activity against CMV [see Microbiology (12.4)].
12.3. Pharmacokinetics
Absorption
At the end of a 1-hour intravenous infusion of 5 mg/kg ganciclovir, total AUC ranged between 22.1 ± 3.2 (n=16) and 26.8 ± 6.1 mcg∙hr/mL (n=16) and Cmax ranged between 8.27 ± 1.02 (n=16) and 9.0 ± 1.4 mcg/mL (n=16).
Distribution
The steady-state volume of distribution of ganciclovir after intravenous administration was 0.74 ± 0.15 L/kg (n=98). Ganciclovir diffuses across the placenta. Cerebrospinal fluid concentrations obtained 0.25 to 5.67 hours post-dose in 3 patients who received 2.5 mg/kg ganciclovir intravenously every 8 hours or every 12 hours ranged from 0.31 to 0.68 mcg/mL, representing 24% to 70% of the respective plasma concentrations. Binding to plasma proteins was 1% to 2% over ganciclovir concentrations of 0.5 and 51 mcg/mL.
Elimination
When administered intravenously, ganciclovir exhibits linear pharmacokinetics over the range of 1.6 to 5.0 mg/kg. Renal excretion of unchanged drug by glomerular filtration and active tubular secretion is the major route of elimination of ganciclovir. In patients with normal renal function, 91.3 ± 5.0% (n=4) of intravenously administered ganciclovir was recovered unmetabolized in the urine. Systemic clearance of intravenously administered ganciclovir was 3.52 ± 0.80 mL/min/kg (n=98) while renal clearance was 3.20 ± 0.80 mL/min/kg (n=47), accounting for 91 ± 11% of the systemic clearance (n=47). Half-life was 3.5 ± 0.9 hours (n=98) following intravenous administration.
Specific Populations
Pharmacokinetics in Patients with Renal Impairment
The pharmacokinetics following intravenous administration of CYTOVENE-IV solution were evaluated in 10 immunocompromised patients with renal impairment who received doses ranging from 1.25 to 5.0 mg/kg. Decreased renal function results in decreased clearance of ganciclovir (Table 7).
Table 7. Ganciclovir Pharmacokinetics in Patients with Renal Impairment:
| Estimated Creatinine Clearance (mL/min) | n | Dose | Clearance (mL/min) Mean ± SD | Half-life (hours) Mean ± SD |
|---|---|---|---|---|
| 50–79 | 4 | 3.2–5 mg/kg | 128 ± 63 | 4.6 ± 1.4 |
| 25–49 | 3 | 3–5 mg/kg | 57 ± 8 | 4.4 ± 0.4 |
| <25 | 3 | 1.25–5 mg/kg | 30 ± 13 | 10.7 ± 5.7 |
Plasma concentrations of ganciclovir are reduced by about 50% during a 4 hour hemodialysis session.
Pharmacokinetics in Geriatric Patients
The pharmacokinetic profiles of CYTOVENE-IV in patients 65 years of age and older have not been established. As ganciclovir is mainly renally excreted and since renal clearance decreases with age, a decrease in ganciclovir total body clearance and a prolongation of ganciclovir half-life can be anticipated in patients 65 years of age and older [see Dosage and Administration (2.5), Use in Specific Populations (8.5)].
Drug Interaction Studies
Table 8 and Table 9 provide a listing of established drug interaction studies with ganciclovir. Table 8 provides the effects of coadministered drug on ganciclovir plasma pharmacokinetic parameters, whereas Table 9 provides the effects of ganciclovir on plasma pharmacokinetic parameters of coadministered drug.
Table 8. Results of Drug Interaction Studies with Ganciclovir: Effects of Coadministered Drug on Ganciclovir Pharmacokinetic Parameters:
| Coadministered Drug | Ganciclovir Dosage | N | Ganciclovir Pharmacokinetic (PK) Parameter |
|---|---|---|---|
| Mycophenolate mofetil (MMF) 1.5 g single dose | 5 mg/kg IV single dose | 12 | No effect on ganciclovir PK parameters observed (patients with normal renal function) |
| rimethoprim 200 mg once daily | 1000 mg orally every 8 hours | 12 | No effect on ganciclovir PK parameters observed. |
| Didanosine 200 mg every 12 hours simultaneously administered with ganciclovir | 5 mg/kg IV twice daily | 11 | No effect on ganciclovir PK parameters observed |
| 5 mg/kg IV once daily | 11 | No effect on ganciclovir PK parameters observed | |
| Probenecid 500 mg every 6 hours | 1000 mg orally every 8 hours | 10 | AUC ↑ 53 ± 91% (range: -14% to 299%) Ganciclovir renal clearance ↓ 22 ± 20% (range: -54% to -4%) |
Table 9. Results of Drug Interaction Studies with Ganciclovir: Effects of Ganciclovir on Pharmacokinetic Parameters of Coadministered Drug:
| Coadministered Drug | Ganciclovir Dosage | N | Coadministered Drug Pharmacokinetic (PK) Parameter |
|---|---|---|---|
| Oral cyclosporine at therapeutic doses | 5 mg/kg infused over 1 hour every 12 hours | 93 | In a retrospective analysis of liver allograft recipients, there was no evidence of an effect on cyclosporine whole blood concentrations. |
| Mycophenolate mofetil (MMF) 1.5 g single dose | 5 mg/kg IV single dose | 12 | No PK interaction observed (patients with normal renal function) |
| Trimethoprim 200 mg once daily | 1000 mg orally every 8 hours | 12 | No effect on trimethoprim PK parameters observed. |
| Didanosine 200 mg every 12 hours | 5 mg/kg IV twice daily | 11 | AUC0-12 ↑70 ± 40% (range: 3% to 121%) Cmax↑49 ± 48% (range: -28% to 125%) |
| Didanosine 200 mg every 12 hours | 5 mg/kg IV once daily | 11 | AUC0-12 ↑50 ± 26% (range: 22% to 110%) Cmax ↑36 ± 36% (range: -27% to 94%) |
12.4. Microbiology
Mechanism of Action
Ganciclovir is a synthetic analogue of 2'-deoxyguanosine, which inhibits replication of human CMV in cell culture and in vivo. In CMV-infected cells, ganciclovir is initially phosphorylated to ganciclovir monophosphate by the viral protein kinase, pUL97. Further phosphorylation occurs by cellular kinases to produce ganciclovir triphosphate, which is then slowly metabolized intracellularly. As the phosphorylation is largely dependent on the viral kinase, phosphorylation of ganciclovir occurs preferentially in virus-infected cells. The virustatic activity of ganciclovir is due to inhibition of the viral DNA polymerase, pUL54, by ganciclovir triphosphate.
Antiviral Activity
The quantitative relationship between the cell culture susceptibility of human herpes viruses to antivirals and clinical response to antiviral therapy has not been established, and virus sensitivity testing has not been standardized. Sensitivity test results, expressed as the concentration of drug required to inhibit the growth of virus in cell culture by 50% (EC50), vary greatly depending upon a number of factors including the assay used. Thus the median concentration of ganciclovir that inhibits CMV replication (EC50 value) in cell culture (laboratory strains or clinical isolates) has ranged from 0.08 to 13.6 µM (0.02 to 3.48 mcg/mL). Ganciclovir inhibits mammalian cell proliferation (CC50 value) in cell culture at higher concentrations ranging from 118 to 2840 µM (30 to 725 mcg/mL). Bone marrow-derived colony-forming cells are more sensitive [CC50 value = 0.1 to 2.7 µM (0.028 to 0.7 mcg/mL)]. The relationship between the antiviral activity in cell culture and clinical response has not been established.
Viral Resistance
Cell Culture: CMV isolates with reduced susceptibility to ganciclovir have been selected in cell culture. Growth of CMV strains in the presence of ganciclovir resulted in the selection of amino acid substitutions in the viral protein kinase pUL97 and the viral DNA polymerase pUL54.
In vivo: Viruses resistant to ganciclovir can arise after prolonged treatment or prophylaxis with ganciclovir by selection of substitutions in pUL97 and/or pUL54. Limited clinical data are available on the development of clinical resistance to ganciclovir and many pathways to resistance likely exist. In clinical isolates, seven canonical pUL97 substitutions, (M460V/I, H520Q, C592G, A594V, L595S, C603W) are the most frequently reported ganciclovir resistance-associated substitutions. These and other substitutions less frequently reported in the literature, or observed in clinical trials, are listed in Table 10.
Table 10. Summary of Resistance-associated Amino Acid Substitutions Observed in the CMV of Patients Failing Ganciclovir Treatment or Prophylaxis:
| pUL97 | L405P, A440V, M460I/V/T/L, V466G/M, C518Y, H520Q, P521L, del 590-593, A591D/V, C592G, A594E/G/T/V/P, L595F/S/T/W, del 595, del 595-603, E596D/G/Y, K599E/M, del 600-601, del 597-600, del 601-603, C603W/R/S/Y, C607F/S/Y, I610T, A613V |
| pUL54 | E315D, N408D/K/S, F412C/L/S, D413A/E/N, L501F/I, T503I, K513E/N/R, D515E, L516W, I521T, P522A/L/S, V526L, C539G, L545S/W, Q578H/L, D588E/N, G629S, S695T, I726T/V, E756K, L773V, V781I, V787L, L802M, A809V, T813S, T821I, A834P, G841A/S, D879G, A972V, del 981-982, A987G |
Note: Many additional pathways to ganciclovir resistance likely exist.
CMV resistance to ganciclovir has been observed in individuals with AIDS and CMV retinitis who have never received ganciclovir therapy. Viral resistance has also been observed in patients receiving prolonged treatment for CMV retinitis with CYTOVENE-IV. In a controlled study of oral ganciclovir for prevention of AIDS-associated CMV disease, 364 individuals had one or more cultures performed after at least 90 days of ganciclovir treatment. Of these, 113 had at least one positive culture. The last available isolate from each subject was tested for reduced sensitivity, and 2 of 40 were found to be resistant to ganciclovir. These resistant isolates were associated with subsequent treatment failure for retinitis.
The possibility of viral resistance should be considered in patients who show poor clinical response or experience persistent viral excretion during therapy.
Cross-Resistance
Cross-resistance has been reported for amino acid substitutions selected in cell culture by ganciclovir, cidofovir or foscarnet. In general, amino acid substitutions in pUL54 conferring cross-resistance to ganciclovir and cidofovir are located within the exonuclease domains and region V of the viral DNA polymerase. Whereas, amino acid substitutions conferring cross-resistance to foscarnet are diverse, but concentrate at and between regions II (codons 696-742) and III (codons 805-845). The amino acid substitutions that resulted in reduced susceptibility to ganciclovir and either cidofovir and/or foscarnet are summarized in Table 11.
Table 11. Summary of pUL54 Amino Acid Substitutions with Cross-resistance between Ganciclovir, Cidofovir, and/or Foscarnet:
| Cross-resistant to cidofovir | D301N, N408D/K, N410K, F412C/L/S/V, D413E/N, P488R, L501I, T503I, K513E/N, L516R/W, I521T, P522S/A, V526L, C539G/R, L545S/W, Q578H, D588N, I726T/V, E756K, L773V, V812L, T813S, A834P, G841A, del 981-982, A987G |
| Cross-resistant to foscarnet | F412C, Q578H/L, D588N, V715A/M, E756K, L773V, V781I, V787L, L802M, A809V, V812L, T813S, T821I, A834P, G841A/S, del 981-982 |
13.1. Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis, Mutagenesis
Ganciclovir was carcinogenic in mice at the same mean drug exposure in humans as at the RHD (5 mg/kg). At the dose of 1000 mg/kg/day (1.4 times the exposure at the RHD), there was a significant increase in the incidence of tumors of the preputial gland in males, forestomach (nonglandular mucosa) in males and females, and reproductive tissues (ovaries, uterus, mammary gland, clitoral gland and vagina) and liver in females. At the dose of 20 mg/kg/day (0.1 times the exposure at the RHD), a slightly increased incidence of tumors was noted in the preputial and harderian glands in males, forestomach in males and females, and liver in females. No carcinogenic effect was observed in mice administered ganciclovir at 1 mg/kg/day (exposure estimated as 0.01 times the RHD). Except for histiocytic sarcoma of the liver, ganciclovir-induced tumors were generally of epithelial or vascular origin. Although the preputial and clitoral glands, forestomach and harderian glands of mice do not have human counterparts, ganciclovir should be considered a potential carcinogen in humans.
Ganciclovir increased mutations in mouse lymphoma cells and DNA damage in human lymphocytes in vitro at concentrations between 50 to 500 and 250 to 2000 µg/mL, respectively. In the mouse micronucleus assay, ganciclovir was clastogenic at doses of 150 and 500 mg/kg (2.8 to 10 times the exposure at the RHD) but not at doses of 50 mg/kg (exposure approximately comparable to the RHD). Ganciclovir was not mutagenic in the Ames Salmonella assay at concentrations of 500 to 5000 µg/mL.
Impairment of Fertility
Ganciclovir caused decreased mating behavior, decreased fertility, and an increased incidence of embryolethality in female mice following doses of 90 mg/kg/day (exposures approximately 1.7 times the RHD). Ganciclovir caused decreased fertility in male mice and hypospermatogenesis in mice and dogs following daily oral or intravenous administration of doses ranging from 0.2 to 10 mg/kg. Systemic drug exposure (AUC) at the lowest dose showing toxicity in each species ranged from 0.03 to 0.1 times the exposure at the RHD.
14. Clinical Studies
14.1 Treatment of CMV Retinitis
In a retrospective, non-randomized, single-center analysis of 41 patients with AIDS and CMV retinitis diagnosed by ophthalmologic examination between August 1983 and April 1988, treatment with CYTOVENE-IV solution resulted in a delay in mean (median) time to first retinitis progression compared to untreated controls [105 (71) days from diagnosis vs 35 (29) days from diagnosis]. Patients in this series received induction treatment of CYTOVENE-IV 5 mg/kg twice daily for 14 to 21 days followed by maintenance treatment with either 5 mg/kg once daily, 7 days per week or 6 mg/kg once daily, 5 days per week.
In a controlled, randomized study conducted between February 1989 and December 1990, immediate treatment with CYTOVENE-IV was compared to delayed treatment in 42 patients with AIDS and peripheral CMV retinitis; 35 of 42 patients (13 in the immediate-treatment group and 22 in the delayed-treatment group) were included in the analysis of time to retinitis progression. Based on masked assessment of fundus photographs, the mean [95% CI] and median [95% CI] times to progression of retinitis were 66 days [39, 94] and 50 days [40, 84], respectively, in the immediate-treatment group compared to 19 days [11, 27] and 13.5 days [8, 18], respectively, in the delayed-treatment group.
Data from trials ICM 1653, ICM 1774, and AVI 034, which were performed comparing CYTOVENE-IV to oral ganciclovir for treatment of CMV retinitis in patients with AIDS, are shown in Table 12 and Figures 1, 2, and 3, and are discussed below.
Table 12. Population Characteristics in Studies ICM 1653, ICM 1774 and AVI 034:
| Demographics | ICM 1653 (n=121) | ICM 1774 (n=225) | AVI 034 (n=159) | |
|---|---|---|---|---|
| Median age (years) Range | 38 24-62 | 37 22-56 | 39 23-62 | |
| Sex | Males | 116 (96%) | 222 (99%) | 148 (93%) |
| Females | 5 (4%) | 3 (1%) | 10 (6%) | |
| Ethnicity | Asian | 3 (3%) | 5 (2%) | 7 (4%) |
| Black | 11 (9%) | 9 (4%) | 3 (2%) | |
| Caucasian | 98 (81%) | 186 (83%) | 140 (88%) | |
| Other | 9 (7%) | 25 (11%) | 8 (5%) | |
| Median CD4 Count Range | 9.5 0-141 | 7.0 0-80 | 10.0 0-320 | |
| Mean (SD) Observation Time (days) | 107.9 (43.0) | 97.6 (42.5) | 80.9 (47.0) | |
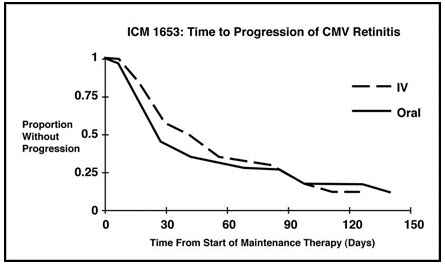
Trial ICM 1653: In this randomized, open-label, parallel group trial, conducted between March 1991 and November 1992, patients with AIDS and newly diagnosed CMV retinitis received a 3-week induction course of CYTOVENE-IV solution, 5 mg/kg twice daily for 14 days followed by 5 mg/kg once daily for 1 additional week. Following the 21-day intravenous induction course, patients with stable CMV retinitis were randomized to receive 20 weeks of maintenance treatment with either CYTOVENE-IV solution, 5 mg/kg once daily, or ganciclovir capsules, 500 mg 6 times daily (3000 mg/day). The study showed that the mean [95% CI] and median [95% CI] times to progression of CMV retinitis, as assessed by masked reading of fundus photographs, were 57 days [44, 70] and 29 days [28, 43], respectively, for patients on oral therapy compared to 62 days [50, 73] and 49 days [29, 61], respectively, for patients on intravenous therapy. The difference [95% CI] in the mean time to progression between the oral and intravenous therapies (oral - IV) was -5 days [-22, 12]. See Figure 1 for comparison of the proportion of patients remaining free of progression over time.
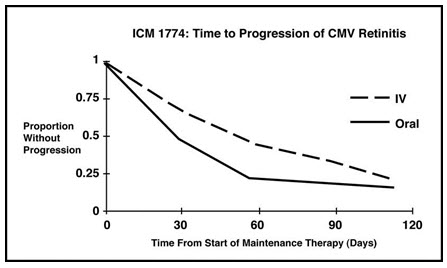
Trial ICM 1774: In this three-arm, randomized, open-label, parallel group trial, conducted between June 1991 and August 1993, patients with AIDS and stable CMV retinitis following from 4 weeks to 4 months of treatment with CYTOVENE-IV solution were randomized to receive maintenance treatment with CYTOVENE-IV solution, 5 mg/kg once daily, ganciclovir capsules, 500 mg 6 times daily, or ganciclovir capsules, 1000 mg three times daily for 20 weeks. The study showed that the mean [95% CI] and median [95% CI] times to progression of CMV retinitis, as assessed by masked reading of fundus photographs, were 54 days [48, 60] and 42 days [31, 54], respectively, for patients on oral therapy compared to 66 days [56, 76] and 54 days [41, 69], respectively, for patients on intravenous therapy. The difference [95% CI] in the mean time to progression between the oral and intravenous therapies (oral - IV) was -12 days [-24, 0]. See Figure 2 for comparison of the proportion of patients remaining free of progression over time.
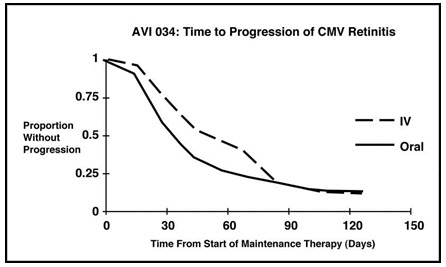
Trial AVI 034: In this randomized, open-label, parallel group trial, conducted between June 1991 and February 1993, patients with AIDS and newly diagnosed (81%) or previously treated (19%) CMV retinitis who had tolerated 10 to 21 days of induction treatment with CYTOVENE-IV, 5 mg/kg twice daily, were randomized to receive 20 weeks of maintenance treatment with either ganciclovir capsules, 500 mg 6 times daily, or CYTOVENE-IV solution, 5 mg/kg/day. The mean [95% CI] and median [95% CI] times to progression of CMV retinitis, as assessed by masked reading of fundus photographs, were 51 days [44, 57] and 41 days [31, 45], respectively, for patients on oral therapy compared to 62 days [52, 72] and 60 days [42, 83], respectively, for patients on intravenous therapy. The difference [95% CI] in the mean time to progression between the oral and intravenous therapies (oral - IV) was -11 days [-24, 1]. See Figure 3 for comparison of the proportion of patients remaining free of progression over time.
Comparison of other CMV retinitis outcomes between oral and IV formulations (development of bilateral retinitis, progression into Zone 1, and deterioration of visual acuity), while not definitive, showed no marked differences between treatment groups in these studies. Because of low event rates among these endpoints, these studies are underpowered to rule out significant differences in these endpoints.
Figure 1. Trial ICM 1653: Time to Progression of CMV Retinitis:
Figure 2. Trial ICM 1774: Time to Progression of CMV Retinitis:
Figure 3. Trial AVI 034: Time to Progression of Retinitis:
14.2 Prevention of CMV Disease in Transplant Recipients
CYTOVENE-IV was evaluated in three randomized, controlled trials of prevention of CMV disease in organ transplant recipients.
Trial ICM 1496: In a randomized, double-blind, placebo-controlled study of 149 heart transplant recipients at risk for CMV infection (CMV seropositive or a seronegative recipient of an organ from a CMV seropositive donor), there was a reduction in the overall incidence of CMV disease in patients treated with CYTOVENE-IV. Immediately post-transplant, patients received CYTOVENE-IV solution 5 mg/kg twice daily for 14 days followed by 6 mg/kg once daily for 5 days/week for an additional 14 days. Twelve of the 76 (16%) patients treated with CYTOVENE-IV vs 31 of the 73 (43%) placebo-treated patients developed CMV disease during the 120-day post-transplant observation period. No significant differences in hematologic toxicities were seen between the two treatment groups [see Adverse Reactions (6.1)].
Trial ICM 1689: In a randomized, double-blind, placebo-controlled study of 72 bone marrow transplant recipients with asymptomatic CMV infection (CMV positive culture of urine, throat or blood) there was a reduction in the incidence of CMV disease in patients treated with CYTOVENE-IV following successful hematopoietic engraftment. Patients with virologic evidence of CMV infection received CYTOVENE-IV solution 5 mg/kg twice daily for 7 days followed by 5 mg/kg once daily through day 100 post-transplant. One of the 37 (3%) patients treated with CYTOVENE-IV vs 15 of the 35 (43%) placebo-treated patients developed CMV disease during the study. At 6 months post-transplant, there continued to be a reduction in the incidence of CMV disease in patients treated with CYTOVENE-IV. Six of 37 (16%) patients treated with CYTOVENE-IV vs 15 of the 35 (43%) placebo-treated patients developed disease through 6 months post-transplant. The overall rate of survival was higher in the group treated with CYTOVENE-IV, both at day 100 and day 180 post-transplant. Although the differences in hematologic toxicities were not statistically significant, the incidence of neutropenia was higher in the group treated with CYTOVENE-IV [see Adverse Reactions (6.1)].
Trial ICM 1570: This was a randomized, unblinded study that evaluated 40 allogeneic bone marrow transplant recipients at risk for CMV disease. Patients underwent bronchoscopy and bronchoalveolar lavage (BAL) on day 35 post-transplant. Patients with histologic, immunologic or virologic evidence of CMV infection in the lung were then randomized to observation or treatment with CYTOVENE-IV solution (5 mg/kg twice daily for 14 days followed by 5 mg/kg once daily 5 days/week until day 120). Four of 20 (20%) patients treated with CYTOVENE-IV and 14 of 20 (70%) control patients developed interstitial pneumonia. The incidence of CMV disease was lower in the group treated with CYTOVENE-IV, consistent with the results observed in ICM 1689.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.