DAPIRING Vaginal delivery system Ref.[50157] Active ingredients: Dapivirine
Source: Health Products Regulatory Authority (ZA) Revision Year: 2022 Publisher: LeBasi Pharmaceuticals (Pty) Ltd, San Domenico Building, Unit 6, Ground Floor, 10 Church Street, Durbanville, 7551
4.1. Therapeutic indications
Reducing the risk of HIV-1 infection via vaginal intercourse in HIV-uninfected women 18 years and older in combination with safer sex practices when oral PrEP is not/cannot be used or is not available.
4.2. Posology and method of administration
Posology
One DapiRing is inserted into the vagina and kept in until replaced every 28 days with a new ring.
To maintain efficacy, a new DapiRing should be inserted immediately after the previous ring is removed.
The DapiRing must be used as directed (see "Method of administration"). If the DapiRing is accidentally expelled or removed, the woman should follow the instructions given below under "Accidental expulsion or removal of the DapiRing".
Special populations
Paediatric population
The safety and efficacy of the DapiRing in children under the age of 18 years have not been established.
Currently available data are described in section 4.8 but no recommendation on a posology can be made.
Method of administration
Vaginal use.
Preparation for inserting the DapiRing
The woman should wash her hands in clean water and dry them before removing the DapiRing from the package.
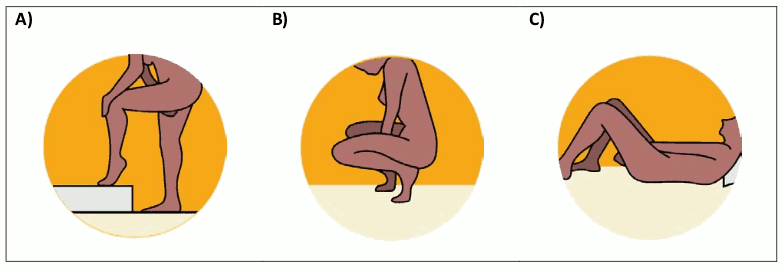
The woman should choose a position that is comfortable for her to insert the DapiRing, for example raising one leg, squatting or lying down (Figure 1A–C).
Figure 1. Positioning for Inserting the DapiRing:
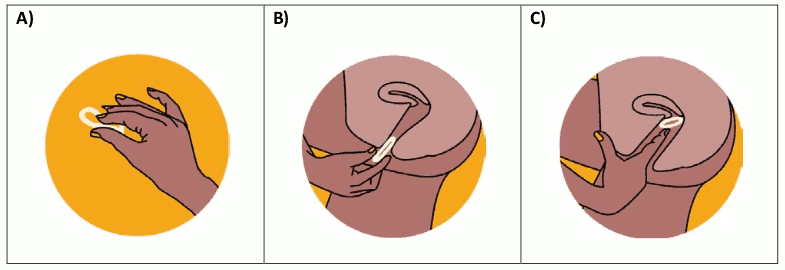
Figure 2. Inserting the DapiRing:
The DapiRing should be held between the thumb and index finger, twisting it into the shape of the number eight (8) or pressing the sides together (Figure 2A). Using the other hand, the folds of the skin around the vagina should be held open. The tip of the ring should be placed in the vagina opening (Figure 2B), and then the index finger should be used to gently push the folded ring into the vagina as far as possible (Figure 2C). If the ring feels uncomfortable, it may not have been pushed far enough into the vagina. In this case, the woman should use her index finger to gently push the ring as far as she can. If the ring still feels uncomfortable, the woman should try reinserting the ring or contacting her healthcare provider. Once the ring is inserted, the woman should wash her hands in clean water and dry them.
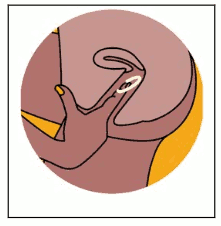
Figure 3. Removing the DapiRing:
The woman should wash her hands in clean water and dry them, choose a comfortable position with her legs apart, and use her finger to hook the ring and gently pull it out of her vagina (Figure 3).
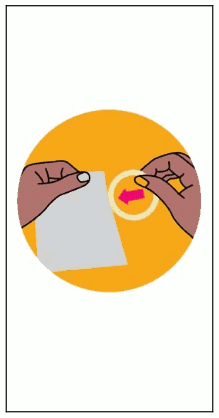
Figure 4. Disposing the Used DapiRing:
To dispose the used DapiRing, the used ring may be placed inside an empty pouch (Figure 4). Alternatively, the used ring may be wrapped in tissue or toilet paper. A refuse bin, which is kept out of reach of children, should be used for disposal. The ring should not be disposed in the toilet (see section 6.6). The woman should wash her hands in clean water and dry them after handling the ring.
Accidental expulsion or removal of the DapiRing
The DapiRing may be accidentally expelled (e.g. during a bowel movement, urination, menses or vaginal intercourse) or removed (e.g. when removing a tampon).
The woman may check if the DapiRing is still in the vagina if she is unsure whether it may have been accidentally expelled. She should follow the instructions for ring removal (Figure 3) to either remove the ring and immediately reinsert it (Figure 2) or choose a comfortable position and insert her index finger into the vagina to feel if the ring is still in place.
If accidental expulsion/removal occurs in a clean environment (e.g. whilst in bed or inside clean clothing), and the DapiRing does not touch an unhygienic surface (e.g. the toilet), the woman may rinse the ring in clean water and immediately re-insert it, as instructed. If the DapiRing touches something unhygienic when accidentally expelled or removed, it should not be re-inserted and should be discarded as instructed (Figure 4). A new ring should be inserted immediately, following the instructions for inserting the DapiRing (Figure 2).
4.9. Overdose
The potential for overdose using the DapiRing is considered highly unlikely. No case of overdose has been reported in clinical trials. If an overdose occurs, standard supportive treatment should be applied as necessary.
6.3. Shelf life
60 months.
Store at or below 30°C.
6.4. Special precautions for storage
Store in the original package in order to protect from light.
6.5. Nature and contents of container
Each DapiRing is packaged into a laminated (PET-Alu/Adhesive/PP), square, heat-sealed pouch.
A carton contains either one pouch (1 ring) or three pouches (3 rings).
6.6. Special precautions for disposal and other handling
Do not use if the seal on the pouch is broken.
Do not flush used or unused product.
Do not throw away the DapiRing in the toilet or water drains. The used DapiRing should either be placed in an empty pouch or wrapped in tissue or toilet paper and disposed in the refuse bin, out of reach of children.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.