DIMENHYDRINATE Solution for injection Ref.[10838] Active ingredients: Dimenhydrinate
Source: FDA, National Drug Code (US) Revision Year: 2019
Product description
Dimenhydrinate, an anti-nauseant/antiemetic, is the 8-chlorotheophylline salt of diphenhydramine. It contains not less than 53% and not more than 55.5% of diphenhydramine, and not less than 44% and not more than 47% of 8-chlorotheophylline, calculated on the dried basis.
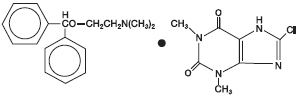
Chemically, it is 8-chlorotheophylline compound with 2(diphenylmethoxy)-N,N-dimethylethylamine (1:1), and the structural formula is:
C17H21NO•C7H7ClN4O2
M.W. 469.96
Dimenhydrinate Injection, USP contains a sterile solution of Dimenhydrinate 50 mg/mL; Propylene Glycol 50%; Benzyl Alcohol 5% as preservative; and Water for Injection q.s. Sodium Hydroxide and/or Hydrochloric Acid may have been used to adjust pH.
| How Supplied | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
DimenhyDRINATE Injection, USP, 50 mg/mL is available in multiple dose amber vials, as follows:
|
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.