DIVIGEL Gel Ref.[10877] Active ingredients: Estradiol
Source: FDA, National Drug Code (US) Revision Year: 2020
Product description
Divigel (estradiol gel) 0.1 percent, is a clear, colorless gel, which is odorless when dry. It is designed to deliver sustained circulating concentrations of estradiol when applied once daily to the skin. The gel is applied to a small area (200 cm2) of the thigh in a thin layer. Divigel is available in five doses of 0.25, 0.5, 0.75, 1.0, and 1.25 grams for topical application (corresponding to 0.25, 0.5, 0.75, 1.0, and 1.25 mg estradiol, respectively).
The active component of the topical gel is estradiol.
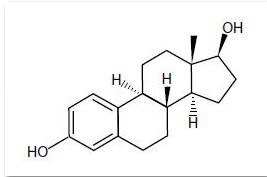
Estradiol is a white crystalline powder, chemically described as estra-1,3,5(10)-triene-3,17ß-diol. It has an empirical formula of C18H24O2 and molecular weight of 272.39. The structural formula is:
The remaining components of the gel (carbomer, ethanol, propylene glycol, purified water, and triethanolamine) are pharmacologically inactive.
| Dosage Forms and Strengths |
|---|
|
Divigel is available in five doses of 0.25, 0.5, 0.75, 1.0, and 1.25 grams for transdermal application (corresponding to 0.25, 0.5, 0.75, 1.0, and 1.25 mg estradiol, respectively). Divigel is a clear, colorless gel, which is odorless when dry. |
| How Supplied |
|---|
|
Divigel (estradiol gel) 0.1% is a clear, colorless, smooth, opalescent gel supplied in single-dose foil packets of 0.25, 0.5, 0.75, 1.0, and 1.25 grams, corresponding to 0.25, 0.5, 0.75, 1.0, and 1.25 mg estradiol, respectively. NDC 68025-065-30, carton of 30 packets, 0.25 mg estradiol per single-dose foil packet NDC 68025-066-30, carton of 30 packets, 0.5 mg estradiol per single-dose foil packet NDC 68025-083-30, carton of 30 packets, 0.75 mg estradiol per single-dose foil packet NDC 68025-067-30, carton of 30 packets, 1.0 mg estradiol per single-dose foil packet NDC 68025-086-30, carton of 30 packets, 1.25 mg estradiol per single-dose foil packet Keep out of the reach of children. |
Drugs
| Drug | Countries | |
|---|---|---|
| DIVIGEL | Canada, Estonia, Finland, Ireland, Japan, Lithuania, Poland, Singapore, United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.