DIVIGEL Gel Ref.[10877] Active ingredients: Estradiol
Source: FDA, National Drug Code (US) Revision Year: 2020
4. Contraindications
Divigel is contraindicated in women with any of the following conditions:
- Undiagnosed abnormal genital bleeding [see Warning and Precautions (5.2)]
- Breast cancer or history of breast cancer [see Warning and Precautions (5.2)]
- Estrogen-dependent neoplasia [see Warning and Precautions (5.2)]
- Active DVT, PE, or history of these conditions [see Warning and Precautions (5.1)]
- Active arterial thromboembolic disease (e.g. stroke and MI), or a history of these conditions [see Warning and Precautions (5.1)]
- Known anaphylactic reaction, angioedema, or hypersensitivity to Divigel
- Hepatic impairment or disease
- Protein C, protein S, or antithrombin deficiency, or other known thrombophilic disorders
5. Warnings and Precautions
5.1 Cardiovascular Disorders
Increased risk of stroke and DVT are reported with estrogen-alone therapy. Increased risk of PE, DVT, stroke and MI are reported with estrogen plus progestin therapy.
Immediately discontinue estrogen with or without progestin therapy if any of these occur or is suspected.
Manage appropriately any risk factors for arterial vascular disease (e.g., hypertension, diabetes mellitus, tobacco use, hypercholesterolemia, and obesity) and/or venous thromboembolism (VTE) (e.g., personal history or family history of VTE, obesity, and systemic lupus erythematosus).
Stroke
The WHI estrogen-alone substudy reported a statistically significant increased risk of stroke in women 50 to 79 years of age receiving daily CE (0.625 mg)-alone compared to women in the same age group receiving placebo (45 versus 33 strokes per 10,000 women-years, respectively). The increase in risk was demonstrated in year 1 and persisted [see Clinical Studies (14.2)]. Immediately discontinue estrogen-alone therapy if a stroke occurs or is suspected.
Subgroup analyses of women 50 to 59 years of age suggest no increased risk of stroke for those women receiving CE (0.625 mg)-alone versus those receiving placebo (18 versus 21 per 10,000 women-years). 1
The WHI estrogen plus progestin substudy reported a statistically significant increased risk of stroke in women 50 to 79 years of age receiving daily CE (0.625 mg) plus MPA (2.5 mg) compared to women in the same age group receiving placebo (33 versus 25 per 10,000 women-years) [see Clinical Studies (14.2)]. The increase in risk was demonstrated after the first year and persisted. 1 Immediately discontinue estrogen plus progestin therapy if a stroke occurs or is suspected.
Coronary Heart Disease
The WHI estrogen-alone substudy reported no overall effect on coronary heart disease (CHD) events (defined as nonfatal MI, silent MI, or CHD death) in women receiving estrogen-alone compared to placebo 2 [see Clinical Studies (14.2)].
Subgroup analyses of women 50 to 59 years of age suggest a statistically non-significant reduction in CHD events (CE [0.625 mg]-alone compared to placebo) in women with less than 10 years since menopause (8 versus 16 per 10,000 women-years). 1
The WHI estrogen plus progestin substudy reported a statistically non-significant increased risk of CHD events in women receiving daily CE (0.625 mg) plus MPA (2.5 mg) compared to women receiving placebo (41 versus 34 per 10,000 women-years). 1 An increase in relative risk was demonstrated in year 1, and a trend toward decreasing relative risk was reported in years 2 through 5 [see Clinical Studies (14.2)].
In postmenopausal women with documented heart disease (n=2,763, average 66.7 years of age), in a controlled clinical trial of secondary prevention of cardiovascular disease [Heart and Estrogen/Progestin Replacement Study (HERS)], treatment with daily CE (0.625 mg) plus MPA (2.5 mg) demonstrated no cardiovascular benefit. During an average follow-up of 4.1 years, treatment with CE plus MPA did not reduce the overall rate of CHD events in postmenopausal women with established coronary heart disease. There were more CHD events in the CE plus MPA-treated group than in the placebo group in year 1, but not during the subsequent years. Two thousand, three hundred and twenty-one (2,321) women from the original HERS trial agreed to participate in an open label extension of HERS, HERS II. Average follow-up in HERS II was an additional 2.7 years, for a total of 6.8 years overall. Rates of CHD events were comparable among women in the CE plus MPA group and the placebo group in HERS, HERS II, and overall.
Venous Thromboembolism
In the WHI estrogen-alone substudy, the risk of VTE (DVT and PE) was increased for women receiving daily CE (0.625 mg)-alone compared to placebo (30 versus 22 per 10,000 women-years), although only the increased risk of DVT reached statistical significance (23 versus 15 per 10,000 women-years). The increase in VTE risk was demonstrated during the first 2 years 3 [see Clinical Studies (14.2)]. Immediately discontinue estrogen-alone therapy if a VTE occurs or is suspected.
In the WHI estrogen plus progestin substudy, a statistically significant 2-fold greater rate of VTE was reported in women receiving daily CE (0.625 mg) plus MPA (2.5 mg) compared to women receiving placebo (35 versus 17 per 10,000 women-years). Statistically significant increases in risk for both DVT (26 versus 13 per 10,000 women-years) and PE (18 versus 8 per 10,000 women-years) were also demonstrated. The increase in VTE risk was demonstrated during the first year and persisted 4 [see Clinical Studies (14.2)]. Immediately discontinue estrogen plus progestin therapy if a VTE occurs or is suspected.
If feasible, discontinue estrogens at least 4 to 6 weeks before surgery of the type associated with an increased risk of thromboembolism, or during periods of prolonged immobilization.
5.2 Malignant Neoplasms
Endometrial Cancer
An increased risk of endometrial cancer has been reported with the use of unopposed estrogen therapy in a woman with a uterus. The reported endometrial cancer risk among unopposed estrogen users is about 2 to 12 times greater than in non-users, and appears dependent on duration of treatment and on estrogen dose. Most studies show no significant increased risk associated with use of estrogens for less than 1 year. The greatest risk appears associated with prolonged use, with increased risk of 15- to 24-fold for 5 to 10 years or more and this risk has been shown to persist for at least 8 to 15 years after estrogen therapy is discontinued.
Clinical surveillance of all women using estrogen-alone or estrogen plus progestin therapy is important. Undertake adequate diagnostic measures, including directed or random endometrial sampling when indicated, to rule out malignancy in postmenopausal women with undiagnosed persistent or recurring abnormal genital bleeding with unknown etiology. There is no evidence that the use of natural estrogens results in a different endometrial risk profile than synthetic estrogens of equivalent estrogen dose. Adding a progestin to postmenopausal estrogen therapy has been shown to reduce the risk of endometrial hyperplasia, which may be a precursor to endometrial cancer.
Breast Cancer
The WHI substudy of daily CE (0.625 mg)-alone provided information about breast cancer in estrogen-alone users. In the WHI estrogen-alone substudy, after an average follow-up of 7.1 years, daily CE-alone was not associated with an increased risk of invasive breast cancer [relative risk (RR) 0.80] compared to placebo 5 [see Clinical Studies (14.2)].
After a mean follow-up of 5.6 years, the WHI substudy of daily CE (0.625 mg) plus MPA (2.5 mg) reported an increased risk of invasive breast cancer in women who took daily CE plus MPA compared to placebo. In this substudy, prior use of estrogen-alone or estrogen plus progestin therapy was reported by 26 percent of the women. The relative risk of invasive breast cancer was 1.24, and the absolute risk was 41 versus 33 cases per 10,000 women-years, for CE plus MPA compared with placebo. Among women who reported prior use of hormone therapy, the relative risk of invasive breast cancer was 1.86, and the absolute risk was 46 versus 25 cases per 10,000 women-years, for CE plus MPA compared with placebo. Among women who reported no prior use of hormone therapy, the relative risk of invasive breast cancer was 1.09, and the absolute risk was 40 versus 36 cases per 10,000 women-years for CE plus MPA compared with placebo. In the same substudy, invasive breast cancers were larger, were more likely to be node positive, and were diagnosed at a more advanced stage in the CE (0.625 mg) plus MPA (2.5 mg) group compared with the placebo group. Metastatic disease was rare, with no apparent difference between the two groups. Other prognostic factors such as histologic subtype, grade and hormone receptor status did not differ between the groups 6 [see Clinical Studies (14.2)].
Consistent with the WHI clinical trial, observational studies have also reported an increased risk of breast cancer with estrogen plus progestin therapy, and a smaller increase in the risk for breast cancer with estrogen-alone therapy, after several years of use. The risk increased with duration of use, and appeared to return to baseline over about 5 years after stopping treatment (only the observational studies have substantial data on risk after stopping). Observational studies also suggest that the risk of breast cancer was greater, and became apparent earlier, with estrogen plus progestin therapy as compared to estrogen-alone therapy.
These studies have not generally found significant variation in the risk of breast cancer among different estrogen plus progestin combinations, doses, or routes of administration.
The use of estrogen-alone and estrogen plus progestin has been reported to result in an increase in abnormal mammograms requiring further evaluation.
All women should receive yearly breast examinations by a healthcare provider and perform monthly breast self-examinations. In addition, mammography examinations should be scheduled based on patient age, risk factors, and prior mammogram results.
Ovarian Cancer
The CE plus MPA substudy of WHI reported that estrogen plus progestin increased the risk of ovarian cancer. After an average follow-up of 5.6 years, the relative risk for CE plus MPA versus placebo was 1.58 [95 percent CI, 0.77–3.24 but was not statistically significant]. The absolute risk for CE plus MPA versus placebo was 4 versus 3 cases per 10,000 women-years. 7
A meta-analysis of 17 prospective and 35 retrospective epidemiology studies found that women who used hormonal therapy for menopausal symptoms had an increased risk for ovarian cancer. The primary analysis, using case-control comparisons, included 12,110 cancer cases from the 17 prospective studies. The relative risks associated with current use of hormonal therapy was 1.41 (95% confidence interval [CI] 1.32 to 1.50); there was no difference in the risk estimates by duration of the exposure (less than 5 years [median of 3 years] vs. greater than 5 years [median of 10 years] of use before the cancer diagnosis). The relative risk associated with combined current and recent use (discontinued use within 5 years before cancer diagnosis) was 1.37 (95% CI 1.27-1.48), and the elevated risk was significant for both estrogen-alone and estrogen plus progestin products. The exact duration of hormone therapy use associated with an increased risk of ovarian cancer, however, is unknown.
5.3 Probable Dementia
In the WHI Memory Study (WHIMS) estrogen-alone ancillary study, a population of 2,947 hysterectomized women 65 to 79 years of age was randomized to daily CE (0.625 mg)-alone or placebo.
After an average follow-up of 5.2 years, 28 women in the estrogen-alone group and 19 women in the placebo group were diagnosed with probable dementia. The relative risk of probable dementia for CE- alone versus placebo was 1.49 (95 percent CI, 0.83–2.66). The absolute risk of probable dementia for CE-alone versus placebo was 37 versus 25 cases per 10,000 women-years 8 [see Use in Specific Populations (8.5), and Clinical Studies (14.3)].
In the WHIMS estrogen plus progestin ancillary study, a population of 4,532 postmenopausal women 65 to 79 years of age was randomized to daily CE (0.625 mg) plus MPA (2.5 mg) or placebo. After an average follow-up of 4 years, 40 women in the CE plus MPA group and 21 women in the placebo group were diagnosed with probable dementia. The relative risk of probable dementia for CE plus MPA versus placebo was 2.05 (95 percent CI, 1.21–3.48). The absolute risk of probable dementia for CE plus MPA versus placebo was 45 versus 22 cases per 10,000 women-years 8 [see Use in Specific Populations (8.5), and Clinical Studies (14.3)].
When data from the two populations in the WHIMS estrogen-alone and estrogen plus progestin ancillary studies were pooled as planned in the WHIMS protocol, the reported overall relative risk for probable dementia was 1.76 (95 percent CI, 1.19–2.60). Since both ancillary studies were conducted in women 65 to 79 years of age, it is unknown whether these findings apply to younger postmenopausal women 8 [see Use in Specific Populations (8.5), and Clinical Studies (14.3)].
5.4 Gallbladder Disease
A 2- to 4-fold increase in the risk of gallbladder disease requiring surgery in postmenopausal women receiving estrogens has been reported.
5.5 Hypercalcemia
Estrogen administration may lead to severe hypercalcemia in women with breast cancer and bone metastases. Discontinue estrogens, including Divigel, if hypercalcemia occurs, and take appropriate measures to reduce the serum calcium level.
5.6 Visual Abnormalities
Retinal vascular thrombosis has been reported in patients receiving estrogens. Discontinue estrogens pending examination if there is sudden partial or complete loss of vision, or a sudden onset of proptosis, diplopia, or migraine. Discontinue estrogens, including Divigel, if examination reveals papilledema or retinal vascular lesions.
5.7 Addition of a Progestin When a Woman Has Not Had a Hysterectomy
Studies of the addition of a progestin for 10 or more days of a cycle of estrogen administration, or daily with estrogen in a continuous regimen, have reported a lowered incidence of endometrial hyperplasia than would be induced by estrogen treatment alone. Endometrial hyperplasia may be a precursor to endometrial cancer.
There are, however, possible risks that may be associated with the use of progestins with estrogens compared to estrogen-alone regimens. These include an increased risk of breast cancer.
5.8 Elevated Blood Pressure
In a small number of case reports, substantial increases in blood pressure have been attributed to idiosyncratic reactions to estrogens. In a large, randomized, placebo-controlled clinical trial, a generalized effect of estrogens on blood pressure was not seen.
5.9 Exacerbation of Hypertriglyceridemia
In women with pre-existing hypertriglyceridemia, estrogen therapy may be associated with elevations of plasma triglycerides leading to pancreatitis. Consider discontinuation of Divigel if pancreatitis occurs.
5.10 Hepatic Impairment and/or Past History of Cholestatic Jaundice
Estrogens may be poorly metabolized in women with hepatic impairment. For women with a history of cholestatic jaundice associated with past estrogen use or with pregnancy, caution should be exercised, and in the case of recurrence of cholestatic jaundice, discontinue Divigel.
5.11 Exacerbation of Hypothyroidism
Estrogen administration leads to increased thyroid-binding globulin (TBG) levels. Women with normal thyroid function can compensate for the increased TBG by making more thyroid hormone, thus maintaining free T4 and T3 serum concentrations in the normal range. Women dependent on thyroid hormone replacement therapy who are also receiving estrogens may require increased doses of their thyroid replacement therapy. Monitor thyroid function in these women during treatment with Divigel to maintain their free thyroid hormone levels in an acceptable range.
5.12 Fluid Retention
Estrogens may cause some degree of fluid retention. Monitor any woman with a condition(s) that might predispose her to fluid retention, such as a cardiac or renal dysfunction. Discontinue estrogen-alone therapy with evidence of medically concerning fluid retention.
5.13 Hypocalcemia
Estrogen-induced hypocalcemia may occur in women with hypoparathyroidism. Consider whether the benefits of estrogen therapy outweigh the risks in such women.
5.14 Exacerbation of Endometriosis
A few cases of malignant transformation of residual endometrial implants have been reported in women treated post-hysterectomy with estrogen-alone therapy. Consider the addition of a progestin for women known to have residual endometriosis post-hysterectomy.
5.15 Hereditary Angioedema
Exogenous estrogens may exacerbate symptoms of angioedema in women with hereditary angioedema. Consider whether the benefits of estrogen therapy outweigh the risks in such women.
5.16 Exacerbation of Other Conditions
Estrogen therapy may cause an exacerbation of asthma, diabetes mellitus, epilepsy, migraine, porphyria, systemic lupus erythematosus, and hepatic hemangiomas. Consider whether the benefits of estrogen therapy outweigh the risks in such women.
5.17 Photosensitivity
The effects of direct sun exposure to Divigel application sites have not been evaluated in clinical trials.
5.18 Application of Sunscreen and Topical Solutions
Studies conducted using other approved topical estrogen gel products have shown that sunscreens have the potential for changing the systemic exposure of topically applied estrogen gels.
The effect of sunscreens and other topical lotions on the systemic exposure of Divigel has not been evaluated in clinical trials.
5.19 Flammability of Alcohol-Based Gels
Alcohol based gels are flammable. Avoid fire, flame, or smoking until the gel has dried.
Occlusion of the area where the topical drug product is applied with clothing or other barriers is not recommended until the gel is completely dried.
5.20 Potential for Estradiol Transfer and Effects of Washing
There is a potential for drug transfer from one individual to the other following physical contact of Divigel application sites. In a study to evaluate transferability to males from their female contacts, there was some elevation of estradiol levels over baseline in the male subjects; however, the degree of transferability in this study was inconclusive. Women are advised to avoid skin contact with other persons until the gel is completely dried. The site of application should be covered (clothed) after drying.
Washing the application site with soap and water 1 hour after application resulted in a 30 to 38 percent decrease in the mean total 24-hour exposure to estradiol. Therefore, women should refrain from washing the application site for at least one hour after application.
5.21 Laboratory Tests
Serum follicle stimulating hormone (FSH) and estradiol levels have not been shown to be useful in the management of moderate to severe vasomotor symptoms.
5.22 Drug-Laboratory Test Interactions
- Accelerated prothrombin time, partial thromboplastin time, and platelet aggregation time; increased platelet count; increased factors II, VII antigen, VIII antigen, VIII coagulant activity, IX, X, XII, VII-X complex, II-VII-X complex, and beta-thromboglobulin; decreased levels of anti-factor Xa and antithrombin III, decreased antithrombin III activity; increased levels of fibrinogen and fibrinogen activity; increased plasminogen antigen and activity.
- Increased thyroid binding globulin (TBG) levels leading to increased circulating total thyroid hormone levels, as measured by protein-bound iodine (PBI), T4 levels (by column or by radioimmunoassay) or T3 levels by radioimmunoassay. T3 resin uptake is decreased, reflecting the elevated TBG. Free T4 and free T3 concentrations are unaltered. Women on thyroid replacement therapy may require higher doses of thyroid hormone.
- Other binding proteins may be elevated in serum, for example, corticosteroid binding globulin (CBG), sex hormone-binding globulin (SHBG), leading to increased total circulating corticosteroids and sex steroids, respectively. Free hormone concentrations, such as testosterone and estradiol, may be decreased. Other plasma proteins may be increased (angiotensinogen/renin substrate, alpha-l-antitrypsin, ceruloplasmin).
- Increased plasma high-density lipoprotein (HDL) and HDL2 cholesterol subfraction concentrations, reduced low-density lipoprotein (LDL) cholesterol concentration, increased triglyceride levels.
- Impaired glucose tolerance.
6. Adverse Reactions
The following serious adverse reactions are discussed elsewhere in the labeling:
- Cardiovascular Disorders [see Boxed Warning, Warnings and Precautions (5.1)].
- Malignant Neoplasms [see Boxed Warning, Warnings and Precautions (5.2)].
6.1. Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
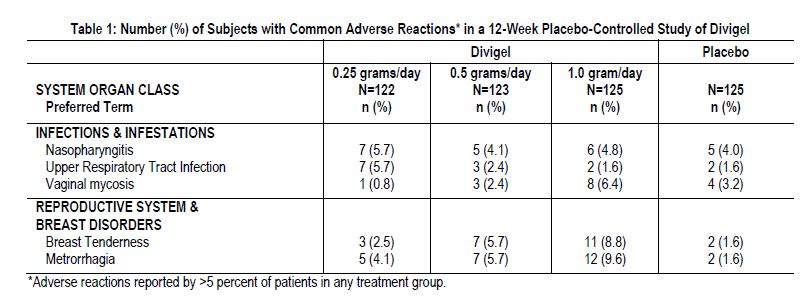
Divigel was studied at doses of 0.25, 0.5 and 1.0 gram per day in a 12-week, double-blind, placebo-controlled study that included a total of 495 postmenopausal women (86.5 percent Caucasian). The adverse reactions that occurred at a rate greater than 5 percent and greater than placebo in any of the treatment groups are summarized in Table 1.
In a 12-week placebo-controlled study of Divigel, application site reactions were seen in <1 percent of participating women.
6.2. Postmarketing Experience
The following adverse reactions have been identified during post-approval use of Divigel. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Genitourinary System: Amenorrhea, dysmenorrhea, ovarian cyst, vaginal discharge
Breasts: Gynecomastia
Cardiovascular: Palpitations, ventricular extrasystoles
Gastrointestinal: Flatulence
Skin: Rash pruritic, urticaria
Eyes: Retinal vein occlusion
Central Nervous System: Tremor
Miscellaneous: Arthralgia, application site rash, asthenia, chest discomfort, fatigue, feeling abnormal, heart rate increased, insomnia, malaise, muscle spasms, pain in extremity, weight increased
7. Drug Interactions
In vitro and in vivo studies have shown that estrogens are metabolized partially by cytochrome P450 3A4 (CYP3A4). Therefore, inducers or inhibitors of CYP3A4 may affect estrogen drug metabolism. Inducers of CYP3A4, such as St. John's wort (Hypericum perforatum) preparations, phenobarbital, carbamazepine, and rifampin, may reduce plasma concentrations of estrogens, possibly resulting in a decrease in therapeutic effects and/or changes in the uterine bleeding profile. Inhibitors of CYP3A4, such as erythromycin, clarithromycin, ketoconazole, itraconazole, ritonavir, and grapefruit juice, may increase plasma concentrations of estrogens and result in adverse reactions.
8.1. Pregnancy
Risk Summary
Divigel is not indicated for use in pregnant women. There are no data with the use of Divigel in pregnant women; however, epidemiologic studies and meta-analyses have not found an increased risk of genital or nongenital birth defects (including cardiac anomalies and limb-reduction defects) following exposure to combined hormonal contraceptives (estrogen and progestins) before conception or during early pregnancy. Animal studies to evaluate embryo/fetal toxicity were not conducted with Divigel.
8.2. Lactation
Risk Summary
Estrogens are present in human milk and can reduce milk production in breast-feeding women. This reduction can occur at any time but is less likely to occur once breast-feeding is well established.
8.4. Pediatric Use
Divigel is not indicated for use in pediatric patients. Clinical studies have not been conducted in the pediatric population.
8.5. Geriatric Use
There have not been sufficient numbers of geriatric women involved in studies utilizing Divigel to determine whether those over 65 years of age differ from younger subjects in their response to Divigel.
The Women's Health Initiative Studies
In the WHI estrogen-alone substudy (daily CE [0.625 mg]-alone versus placebo), there was a higher relative risk of stroke in women greater than 65 years of age [see Clinical Studies (14.2)].
In the WHI estrogen plus progestin substudy (daily CE [0.625 mg] plus MPA [2.5 mg] versus placebo), there was a higher relative risk of nonfatal stroke and invasive breast cancer in women greater than 65 years of age [see Clinical Studies (14.2)].
The Women's Health Initiative Memory Study
In the WHIMS ancillary studies of postmenopausal women 65 to 79 years of age, there was an increased risk of developing probable dementia in women receiving estrogen-alone or estrogen plus progestin when compared to placebo [see Warnings and Precautions (5.3), and Clinical Studies (14.3)].
Since both ancillary studies were conducted in women 65 to 79 years of age, it is unknown whether these findings apply to younger postmenopausal women8 [see Warnings and Precautions (5.3), and Clinical Studies (14.3)]/em>.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.