DOPTELET Film-coated tablet Ref.[27846] Active ingredients: Avatrombopag
Source: European Medicines Agency (EU) Revision Year: 2021 Publisher: Swedish Orphan Biovitrum AB (publ), SE-112 76 Stockholm, Sweden
5.1. Pharmacodynamic properties
Pharmacotherapeutic group: Antihemorrhagics, other systemic hemostatics
ATC code: B02BX08
Mechanism of action
Avatrombopag is an orally active, small molecule thrombopoietin (TPO) receptor agonist that stimulates proliferation and differentiation of megakaryocytes from bone marrow progenitor cells resulting in increased production of platelets. Avatrombopag does not compete with TPO for binding to the TPO receptor and has an additive effect with TPO on platelet production.
Clinical efficacy and safety
Chronic liver disease studies
The efficacy and safety of avatrombopag for the treatment of adult patients with chronic liver disease and a platelet count ˂50 x 109/L who were scheduled to undergo a procedure were studied in 2 identically-designed multicenter, randomised, double-blind, placebo-controlled Phase 3 studies (ADAPT-1 and ADAPT-2). In each study, patients were assigned to the low baseline platelet count cohort (˂40 x 109/L) or the high baseline platelet count cohort (≥40 to ˂50 x 109/L) based on their platelet count at baseline. Patients were then randomised 2:1 to either avatrombopag or placebo.
Patients in the low baseline platelet count cohort received 60 mg avatrombopag or matching placebo once daily for 5 days, and patients in the high baseline platelet count cohort received 40 mg avatrombopag or matching placebo once daily for 5 days. Eligible patients were scheduled to undergo their procedure (low bleeding risk procedures, such as endoscopy and colonoscopy (60.8%); moderate bleeding risk, such as liver biopsy and chemoembolization for HCC (17.2%); or high bleeding risk, such as dental procedures and radiofrequency ablation (22.1%)) 5 to 8 days after their last dose of treatment. Patient populations were similar between the low and high baseline platelet count cohorts, and consisted of 66% male and 35% female; median age 58 years and 61% White, 34% Asian, and 3% Black. A total of 24.8% of patients were ≥65 years of age, 4.6% ≥75 years of age, and only 1 (0.2%) ≥85 years of age. Patients' MELD scores ranged from <10 (37.5%), 10 to 14 (46.3%) and from >14 to <24 (16.2%), and included patients with CTP Class A (56.4%), Class B (38.1%), and Class C (5.6%).
In ADAPT-1, a total of 231 patients were randomised; 149 patients to the avatrombopag group and 82 patients to the placebo group. In the low baseline platelet count cohort, the mean baseline platelet count for the avatrombopag-treated group was 31.1 x 109/L and for placebo-treated patients was 30.7 x 109/L. In the high baseline platelet count cohort, the mean baseline platelet count for the avatrombopag-treated patients was 44.3 x 109/L and for placebo-treated patients was 44.9 x 109/L.
In ADAPT-2, a total of 204 patients were randomised; 128 patients to the avatrombopag treatment group and 76 patients to the placebo treatment group. In the low baseline platelet count cohort, the mean baseline platelet count for the avatrombopag-treated group was 32.7 x 109/L and for placebo-treated patients was 32.5 x 109/L. In the high baseline platelet count cohort, the mean baseline platelet count for the avatrombopag-treated patients was 44.3 x 109/L and for placebo-treated patients was 44.5 x 109/L.
Responders were defined as patients who did not require a platelet transfusion or any rescue procedure for bleeding after randomisation and up to 7 days following a scheduled procedure. Results are shown in Table 5.
Table 5. Efficacy results by baseline platelet count cohort and treatment group – ADAPT-1 and ADAPT-2:
| Low baseline platelet count cohort (<40 x 109/l) | ||||
|---|---|---|---|---|
| Category | ADAPT-1 | ADAPT-2 | ||
| Placebo (n=48) | Avatrombopag 60 mg (n=90) | Placebo (n=43) | Avatrombopag 60 mg (n=70) | |
| Proportion of subjects not requiring a platelet transfusion or rescue procedure for bleeding | ||||
| Responders 95% CIa | 23% (11, 35) | 66% (56, 75) | 35% (21, 49) | 69% (58, 79) |
| P-valueb | <0.0001 | 0.0006 | ||
| Proportion of subjects who achieved a platelet count ≥50 × 109/L on procedure day | ||||
| Responders 95% CIa | 4% (0, 10) | 69% (59, 79) | 7% (0, 15) | 67% (56, 78) |
| P-valueb | <0,0001 | <0,0001 | ||
| Change in platelet count from baseline to procedure day | ||||
| Mean (SD) x 109/l | 0.8 (6.4) | 32.0 (25.5) | 3.0 (10.0) | 31.3 (24.1) |
| Median x 109/l | 0.5 | 28.3 | 0.5 | 28.0 |
| P-valuec | <0.0001 | <0.0001 | ||
| High baseline platelet count (≥40 to <50 x 109/l) | ||||
|---|---|---|---|---|
| Category | ADAPT-1 | ADAPT-2 | ||
| Placebo (n=34) | Avatrombopag 40 mg (n=59) | Placebo (n=33) | Avatrombopag 40 mg (n=58) | |
| Proportion of subjects not requiring a platelet transfusion or rescue procedure for bleeding | ||||
| Responders 95% CIa | 38% (22, 55) | 88% (80, 96) | 33% (17, 49) | 88% (80, 96) |
| P-valueb | <0.0001 | <0.0001 | ||
| Proportion of subjects who achieved a platelet count ≥50 × 109/L on procedure day | ||||
| Responders 95% CIa | 21% (7, 34) | 88% (80, 96) | 39% (23, 56) | 93% (87, 100) |
| P-valueb | <0.0001 | <0.0001 | ||
| Change in platelet count from baseline to procedure day | ||||
| Mean (SD) x 109/l | 1.0 (9.3) | 37.1 (27.4) | 5.9 (14.9) | 44.9 (33.0) |
| Median x 109/l | 0.0 | 33.0 | 3.3 | 41.3 |
| P-valuec | <0.0001 | <0.0001 | ||
a Two-sided 95% confidence interval based on normal approximation.
b Cochran-Mantel-Haenszel Test.
c Wilcoxon Rank Sum Test.
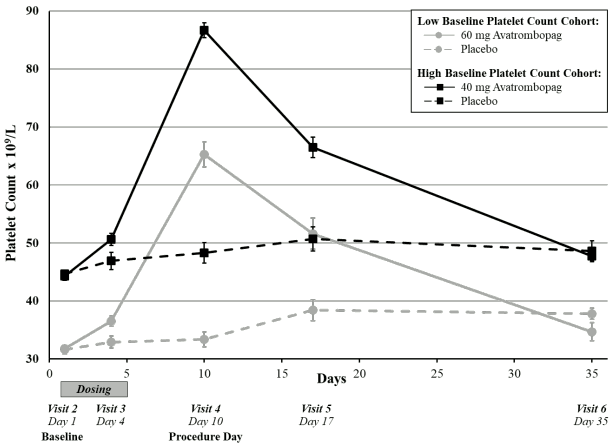
A measured increase in platelet counts was observed in both avatrombopag treatment groups over time beginning on Day 4 post-dose, which peaked on Day 10-13 and then returned to near baseline values by Day 35 (Figure 1); mean platelet count remained greater than or equal to 50 x 109/L on Day 17 (Visit 5).
Figure 1. Mean platelet count (+/- standard error) by days from start of dosing by baseline platelet count cohort and treatment group - pooled ADAPT-1 and ADAPT-2:
The efficacy of avatrombopag was similar across various subgroups for the pooled Phase 3 study population (ADAPT-1 and ADAPT-2). The proportion of subjects not requiring a platelet transfusion or any rescue procedure for bleeding was generally similar across the various subgroups.
Chronic immune thrombocytopenia studies
The efficacy of Doptelet in adult patients with chronic immune thrombocytopenia was evaluated in a Phase 3, multicentre, randomised, double-blind, placebo-controlled trial (Study 302). Patients had previously received one or more prior chronic immune thrombocytopenia therapies and had an average of screening and baseline platelet counts <30 x 109/L. Patients were centrally stratified by splenectomy status, baseline platelet count (≤15 or >15 x 109/L), and use of concomitant chronic immune thrombocytopenia medication, and then randomised (2:1) to receive either avatrombopag or placebo for 6 months. Patients received a starting dose of 20 mg once daily, with doses subsequently titrated based on platelet response.
In addition, patients could taper off concomitant ITP medicinal products and receive rescue treatments as dictated by local standard of care. More than half of all patients in each treatment group had ≥3 prior ITP therapies and 29% of placebo patients and 34% of avatrombopag patients had a prior splenectomy.
Forty-nine patients were randomised, 32 to avatrombopag and 17 to placebo, with similar mean [SD] baseline platelet counts in the 2 treatment groups (14.1 [8.6] x 109/L and 12.7 [7.8] x 109/L, respectively). The median age was 44 years, 63% were female, and 94% were Caucasian, 4% Asian and 2% Black. A total of 8.2% of patients were ≥65 years of age, and no patients were ≥75 years of age. The median duration of exposure was 26 weeks for avatrombopag-treated patients and 6 weeks for placebo-treated patients. The primary efficacy outcome in this trial was the cumulative number of weeks in which the platelet count was ≥50 x 109/L during the 6-month treatment period in the absence of rescue therapy. Avatrombopag-treated patients had a longer duration of platelet counts ≥50 x 109/L in the absence of rescue therapy than those who received placebo (median 12.4 [0, 25] vs 0 [0, 2] weeks, respectively, p<0.0001) (see Table 6).
Table 6. Cumulative number of weeks of platelet response - Study 302:
| Primary efficacy outcome | Avatrombopag (n=32) | Placebo (n=17) |
|---|---|---|
| Cumulative number of weeks with a platelet response* | ||
| Mean (SD) | 12.0 (8.75) | 0.1 (0.49) |
| Median | 12.4 | 0.0 |
| Min, Max | 0, 25 | 0, 2 |
| p-value of Wilcoxon rank sum test | <0.0001 | |
* Cumulative number of weeks of platelet response is defined as the total numbers of weeks in which the platelet count was ≥50 x 109/L during 6 months of treatment in the absence of rescue therapy.
In addition, a larger proportion of patients in the avatrombopag treatment group had platelet counts ≥50 x 109/L at Day 8 compared to placebo (21/32; 66% vs 0/17; 0.0%, respectively; 95% CI (47, 86); p<0.0001). Though few subjects were receiving concomitant ITP medications at baseline, a larger proportion of patients in the avatrombopag treatment group had a reduction in use of concomitant ITP medications from baseline compared to placebo (5/15; 33% vs 0/7; 0.0%, respectively; 95% CI (12, 62); p=0.1348).
Paediatric population
The European Medicines Agency has waived the obligation to submit the results of studies with Doptelet in all subsets of the paediatric population in thrombocytopenia secondary to liver disease (see section 4.2 for information on paediatric use).
5.2. Pharmacokinetic properties
Absorption
The plasma concentration-time profiles following the oral administration of avatrombopag were characterised by a short lag time (0.5–0.75 hours) with peak exposure at 6–8 hours post dose. In a multiple-dose pharmacokinetic study in healthy volunteers, steady state was reached by day 5 of dosing. Open label, randomised, cross-over replicate design clinical trials were conducted in healthy subjects to assess the effects of high-fat and low-fat food on the bioavailability and pharmacokinetic variability of avatrombopag. Administration with either type of food did not have any clinically important effects on rate (Cmax) or extent (AUC) of avatrombopag exposure. However, there was a significant reduction (by approximately 50%) in the between- and within -subject variability of avatrombopag AUC and Cmax when administered with food (see sections 4.2 and 4.5).
Food interaction
Coadministration of avatrombopag with either a high-fat or low-fat meal did not result in clinically important changes in rate or extent of absorption of avatrombopag. However, administration of avatrombopag with both a high and low-fat meal reduced intersubject and intrasubject pharmacokinetic variability of avatrombopag by approximately 50%. Therefore, avatrombopag is recommended to be administered with food (see section 4.2).
Distribution
In vitro studies suggest that avatrombopag is highly bound to human plasma proteins (>96%). The apparent volume of distribution of avatrombopag in patients with thrombocytopenia and chronic liver disease based on population pharmacokinetic analysis is approximately 180 L, and the apparent volume of distribution with patients with chronic immune thrombocytopenia is approximately 235 L, suggesting that avatrombopag is extensively distributed.
Biotransformation
The oxidative metabolism of avatrombopag is mainly mediated by CYP2C9 and CYP3A4/5. Avatrombopag is a substrate for p-glycoprotein (P-gp) mediated transport, although no clinically important differences in platelet count elevations are expected when avatrombopag is co-administered with a strong P-gp inhibitor. Based on in vitro studies, no other transporting proteins (OATP1B1, OATP1B3, OCT2, OAT1, and OAT3) are expected to play a significant role in the disposition of avatrombopag.
Table 7. Drug interactions: Changes in pharmacokinetics of avatrombopag in the presence of co-administered drug:
| Co-administered drug* | Geometric mean ratio [90% CI] of avatrombopag PK with/without co administered drug (No Effect = 1.00) | |
|---|---|---|
| AUC0-inf | Cmax | |
| Strong CYP3A inhibitor | ||
| Itraconazole | 1.37 (1.10, 1.72) | 1.07 (0.86, 1.35) |
| Moderate CYP3A and CYP2C9 inhibitor | ||
| Fluconazole | 2.16 (1.71, 2.72) | 1.17 (0.96, 1.42) |
| Moderate CYP2C9 and strong CYP3A inducer | ||
| Rifampin | 0.57 (0.47, 0.62) | 1.04 (0.88, 1.23) |
| P-gp inhibitor | ||
| Cyclosporine | 0.83 (0.65, 1.04) | 0.66 (0.54, 0.82) |
| P-gp and moderate CYP3A inhibitor | ||
| Verapamil | 1.61 (1.21, 2.15) | 1.26 (0.96, 1.66) |
* at steady state, except for cyclosporine which was administered as a single dose
Effect of avatrombopag
Avatrombopag does not inhibit CYP1A, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, CYP2E1, or CYP3A, does not induce CYP1A, CYP2B6, CYP2C, and CYP3A, and weakly induces CYP2C8 and CYP2C9 in vitro.
Avatrombopag inhibits organic anion transporter (OAT) 1 and 3 and breast cancer resistance protein (BCRP) but not organic anion transporter polypeptide (OATP) 1B1 and 1B3, and organic cation transporter (OCT) 2 in vitro.
Effect of transporting proteins
Avatrombopag is a substrate for P-glycoprotein (P-gp) mediated transport (see Table 7). Avatrombopag is not a substrate for OATP1B1, OATP1B3, OCT2, OAT1, and OAT3.
Elimination
The predominant route of avatrombopag excretion is via faeces. Following administration of a single 20 mg 14C-avatrombopag dose to healthy male volunteers, 88% of the dose was recovered in faeces and 6% in urine. Of the 88% of drug-related material in the faeces, 77% was identified as parent (34%) and the 4-hydroxy metabolite (44%). No metabolites of avatrombopag were detected in plasma.
The mean plasma elimination half-life (%CV) of avatrombopag is approximately 19 hours (19%). The mean (%CV) of the clearance of avatrombopag is estimated to be 6.9 L/hr (29%).
Linearity
Avatrombopag demonstrated dose-proportional pharmacokinetics after single doses from 10 mg (0.5-times the lowest approved dosage) to 80 mg (1.3-times the highest recommended dosage).
Special populations
Elderly
Population pharmacokinetic analysis of avatrombopag plasma concentrations from clinical studies with healthy subjects and patients with thrombocytopenia due to chronic liver disease or healthy subjects and patients with ITP, that included 11% (84/787) and 4% (24/577) of the study population ≥65 years of age, respectively, suggested that avatrombopag exposures are not affected by age (see section 4.2).
Racial or Ethnic Groups
Population pharmacokinetic analysis of avatrombopag plasma concentrations from the clinical studies with healthy subjects, patients with thrombocytopenia due to chronic liver disease, and patients with ITP indicated that avatrombopag exposures were similar across the different races studied.
Renal impairment
Human studies demonstrated that the renal route is not a major pathway for either unchanged avatrombopag or its metabolite's elimination. Based on the known metabolic profile of avatrombopag and the fact that only 6% of the dose is excreted in urine, the likelihood of effects of renal impairment on pharmacokinetics of avatrombopag is considered to be very low (see sections 4.2 and 4.8). The population pharmacokinetic analysis of avatrombopag in healthy subjects and subjects with thrombocytopenia due to chronic liver disease indicated similar exposures between healthy subjects and subjects with mild and moderate renal impairment (CrCL ≥30 mL/min, Cockcroft-Gault).
Pharmacokinetics and pharmacodynamics of avatrombopag have not been investigated in patients with severe renal impairment (CrCL <30 mL/min, Cockcroft-Gault) including patients requiring haemodialysis.
Hepatic impairment
A population pharmacokinetic analysis evaluated avatrombopag plasma exposures in patients with mild to moderate hepatic impairment based on Model for End-Stage Liver Disease (MELD) scores and Child-Turcotte-Pugh scores. No clinically important difference in avatrombopag exposures were observed between patients with Child-Turcotte-Pugh Scores (Range = 5 to 12) or MELD scores (Range = 4 to 23) and healthy subjects. Avatrombopag plasma exposure was comparable in patients with chronic liver disease secondary to viral hepatitis (n=242), non-alcoholic steatohepatitis (n=45) and alcoholic liver disease (n=49) in the pivotal Phase 3 studies, and also comparable to that in healthy subjects (n=391). Due to the limited information available, avatrombopag should only be used in Child-Pugh class C patients when the expected benefit outweighs the expected risks.
5.3. Preclinical safety data
Avatrombopag does not stimulate platelet production in mice, rats, monkeys, or dogs because of the unique TPO receptor specificity. Therefore, data from these animal studies do not fully model potential adverse effects related to platelet count increases due to avatrombopag in humans.
Effects in non-clinical studies were observed only at exposures considered sufficiently in excess of the maximum human exposure indicating little relevance to clinical use. The primary toxicity of avatrombopag in pivotal repeated-dose studies was in the stomach at high doses with adequate safety margins when compared to the exposure at the maximum recommended human dose; these effects were reversible even in the chronic toxicity studies.
Carcinogenesis
In two-year carcinogenicity studies in mice and rats, neuroendocrine cell (enterochromaffin-like cell, ECL cell) gastric tumours (carcinoids) occurred in the stomach at high doses. The gastric carcinoids were considered likely due to prolonged hypergastrinemia observed in toxicity studies. Hypergastrinemia-related gastric carcinoids in rodents are generally considered to be of low risk or relevance to humans.
Avatrombopag was not mutagenic in an in vitro bacterial reverse mutation (AMES) assay or clastogenic in an in vitro human lymphocyte chromosomal aberrations assay or in an in vivo rat bone marrow micronucleus assay.
Animal toxicology and/or pharmacology
In 4-week or longer repeated-dose toxicity studies, treatment-related gastric lesions were observed in mice, rats, and cynomolgus monkeys. In these species, avatrombopag was associated with histopathologic changes in the fundic mucosa of the glandular stomach, characterised by degeneration of the glandular epithelium with a decrease in matured parietal cells. This effect was not associated with inflammatory response or any evidence of erosion or ulcer formation. The severity of gastric lesions was dependent on the dose and duration of avatrombopag administration and showed a clear trend towards reversibility during the recovery period. The exposures (AUC) at doses that showed no gastric lesions across the species were 3- to 33-fold higher than the exposures in humans at the maximum recommended human dose (MRHD).
Reproductive and developmental toxicity
Avatrombopag did not affect fertility or early embryonic development in male rats at exposures 22-times, or in female rats at exposures 114-times, the AUC observed in patients at the recommended dose of 60 mg once daily.
Excretion in milk
Avatrombopag was present in milk of lactating rats after oral administration of radioactive labeled avatrombopag. The pharmacokinetic parameters of avatrombopag in milk were similar to those in plasma with an exposure ratio of avatrombopag-related radioactivity (milk to plasma) of 0.94.
Juvenile animal studies
In a 10-week juvenile toxicology study in rats, avatrombopag was administered at doses ranging from 20 to 300 mg/kg/day. There were no test article-related mortality or clinical signs at doses up to 300 mg/kg/day. In the stomach, dose-dependent degeneration, regenerative hyperplasia, and atrophy of the glandular epithelium occurred at 100 and 300 mg/kg/day; exposures at 100 mg/kg/day in male rats were 14-times the AUC in patients at the maximum recommended dose of 60 mg once daily. Avatrombopag did not cause gastric changes in male juvenile rats at exposures 7 times the AUC observed in patients at the maximum recommended dose of 60 mg once daily. An increased incidence of background focal mineralization was also observed in the kidneys of females at 300 mg/kg/day (female rat exposure was 50-times the human exposure based on AUC at the 60 mg daily dose).
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.