ENABLEX Extended-release tablet Ref.[10803] Active ingredients: Darifenacin
Source: FDA, National Drug Code (US) Revision Year: 2016
Product description
Enablex is an extended-release tablet for oral administration which contains 7.5 mg or 15 mg darifenacin as its hydrobromide salt. The active moiety, darifenacin, is a potent muscarinic receptor antagonist.
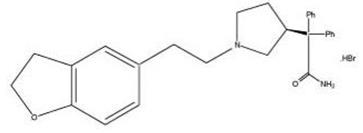
Chemically, darifenacin hydrobromide is (S)2{1-[2-(2,3-dihydrobenzofuran-5-yl)ethyl]-3-pyrrolidinyl}-2,2-diphenylacetamide hydrobromide. The empirical formula of darifenacin hydrobromide is C28H30N2O2•HBr.
The structural formula is:
Darifenacin hydrobromide is a white to almost white, crystalline powder, with a molecular weight of 507.5.
Enablex is a once-a-day extended-release tablet and contains the following inactive ingredients: dibasic calcium phosphate anhydrous, hypromellose, magnesium stearate, polyethylene glycol, talc, titanium dioxide. The 15 mg tablet also contains ferric oxide red and ferric oxide yellow.
| Dosage Forms and Strengths |
|---|
|
Enablex extended-release tablets 7.5 mg are round, shallow, bi-convex, white-colored tablets, and are identified with “DF” on one side and “7.5” on the reverse. Enablex extended-release tablets 15 mg are round, shallow, bi-convex, light peach-colored tablets, and are identified with “DF” on one side and “15” on the reverse. |
| How Supplied |
|---|
|
Enablex, 7.5 mg are round, shallow, bi-convex, white-colored tablets, and are identified with “DF” on one side and “7.5” on the reverse. Bottle of 30 – NDC 0430-0170-15 Enablex, 15 mg are round, shallow, bi-convex, light peach-colored tablets, and are identified with “DF” on one side and “15” on the reverse. Bottle of 30 – NDC 0430-0171-15 Distributed by: Allergan USA, Inc., Irvine, CA 92612 |
Drugs
| Drug | Countries | |
|---|---|---|
| ENABLEX | Brazil, Canada, Ecuador, Hong Kong, New Zealand, United States, South Africa |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.