ENABLEX Extended-release tablet Ref.[10803] Active ingredients: Darifenacin
Source: FDA, National Drug Code (US) Revision Year: 2016
12.1. Mechanism of Action
Darifenacin is a competitive muscarinic receptor antagonist. Muscarinic receptors play an important role in several major cholinergically mediated functions, including contractions of the urinary bladder smooth muscle and stimulation of salivary secretion.
In vitro studies using human recombinant muscarinic receptor subtypes show that darifenacin has greater affinity for the M3 receptor than for the other known muscarinic receptors (9- and 12-fold greater affinity for M3 compared to M1 and M5, respectively, and 59-fold greater affinity for M3 compared to both M2 and M4). M3 receptors are involved in contraction of human bladder and gastrointestinal smooth muscle, saliva production, and iris sphincter function. Adverse drug effects such as dry mouth, constipation and abnormal vision may be mediated through effects on M3 receptors in these organs.
12.2. Pharmacodynamics
In three cystometric studies performed in patients with involuntary detrusor contractions, increased bladder capacity was demonstrated by an increased volume threshold for unstable contractions and diminished frequency of unstable detrusor contractions after Enablex treatment. These findings are consistent with an antimuscarinic action on the urinary bladder.
Electrophysiology
The effect of a six-day treatment of 15 mg and 75 mg Enablex on QT/QTc interval was evaluated in a multiple-dose, double-blind, randomized, placebo- and active-controlled (moxifloxacin 400 mg) parallel-arm design study in 179 healthy adults (44% male, 56% female) aged 18 to 65. Subjects included 18% poor metabolizers (PMs) and 82% extensive metabolizers (EMs). The QT interval was measured over a 24-hour period both predosing and at steady-state. The 75 mg Enablex dose was chosen because this achieves exposure similar to that observed in CYP2D6 poor metabolizers administered the highest recommended dose (15 mg) of darifenacin in the presence of a potent CYP3A4 inhibitor. At the doses studied, Enablex did not result in QT/QTc interval prolongation at any time during the steady-state, while moxifloxacin treatment resulted in a mean increase from baseline QTcF of about 7.0 msec when compared to placebo. In this study, darifenacin 15 mg and 75 mg doses demonstrated a mean heart rate change of 3.1 and 1.3 bpm, respectively, when compared to placebo. However, in the clinical efficacy and safety studies, the change in median HR following treatment with Enablex was no different from placebo.
12.3. Pharmacokinetics
Absorption
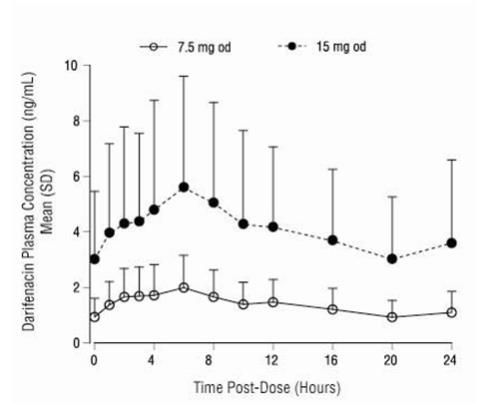
After oral administration of Enablex to healthy volunteers, peak plasma concentrations of darifenacin are reached approximately seven hours after multiple dosing and steady-state plasma concentrations are achieved by the sixth day of dosing. The mean (SD) steady-state time course of Enablex 7.5 mg and 15 mg extended-release tablets is depicted in Figure 1.
* Includes 95 EMs and 6 PMs for 7.5 mg; 104 EMs and 10 PMs for 15 mg.
A summary of mean (standard deviation, SD) steady-state pharmacokinetic parameters of Enablex 7.5 mg and 15 mg extended-release tablets in EMs and PMs of CYP2D6 is provided in Table 3.
Table 3. Mean (SD) Steady-State Pharmacokinetic Parameters from Enablex 7.5 mg and 15 mg Extended-Release Tablets Based on Pooled Data by Predicted CYP2D6 Phenotype:
| Enablex 7.5 mg (N=68 EM, 5 PM) | Enablex 15 mg (N=102 EM, 17 PM) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| AUC24 (ng•h/mL) | Cmax (ng/mL) | Cavg (ng/mL) | Tmax (h) | t1/2 (h) | AUC24 (ng•h/mL) | Cmax (ng/mL) | Cavg (ng/mL) | Tmax (h) | t1/2 (h) | ||
| EM | 29.24 (15.47) | 2.01 (1.04) | 1.22 (0.64) | 6.49 (4.19) | 12.43 (5.64)a | 88.90 (67.87) | 5.76 (4.24) | 3.70 (2.83) | 7.61 (5.06) | 12.05 (12.37)b | |
| PM | 67.56 (13.13) | 4.27 (0.98) | 2.81 (0.55) | 5.20 (1.79) | 19.95c - | 157.71 (77.08) | 9.99 (5.09) | 6.58 (3.22) | 6.71 (3.58) | 7.40 d - | |
a N=25; bN = 8; cN = 2; dN = 1; AUC24 = Area under the plasma concentration versus time curve for 24h;
Cmax = Maximum observed plasma concentration; Cavg = Average plasma concentration at steady-state;
Tmax = Time of occurrence of Cmax; t1/2 = Terminal elimination half-life. Regarding EM and PM [see Clinical Pharmacology, Pharmacokinetics, Variability in Metabolism (12.3)].
The mean oral bioavailability of Enablex in EMs at steady-state is estimated to be 15% and 19% for 7.5 mg and 15 mg tablets, respectively.
Effect of Food
Following single dose administration of Enablex with food, the AUC of darifenacin was not affected, while the Cmax was increased by 22% and Tmax was shortened by 3.3 hours. There is no effect of food on multiple-dose pharmacokinetics from Enablex.
Distribution
Darifenacin is approximately 98% bound to plasma proteins (primarily to alpha-1-acid-glycoprotein). The steady-state volume of distribution (Vss) is estimated to be 163 L.
Metabolism
Darifenacin is extensively metabolized by the liver following oral dosing.
Metabolism is mediated by cytochrome P450 enzymes CYP2D6 and CYP3A4. The three main metabolic routes are as follows:
(i) monohydroxylation in the dihydrobenzofuran ring;
(ii) dihydrobenzofuran ring opening;
(iii) N-dealkylation of the pyrrolidine nitrogen.
The initial products of the hydroxylation and N-dealkylation pathways are the major circulating metabolites but they are unlikely to contribute significantly to the overall clinical effect of darifenacin.
Variability in Metabolism
A subset of individuals (approximately 7% Caucasians and 2% African Americans) are poor metabolizers (PMs) of CYP2D6 metabolized drugs. Individuals with normal CYP2D6 activity are referred to as extensive metabolizers (EMs). The metabolism of darifenacin in PMs will be principally mediated via CYP3A4. The darifenacin ratios (PM versus EM) for Cmax and AUC following darifenacin 15 mg once daily at steady-state were 1.9 and 1.7, respectively.
Excretion
Following administration of an oral dose of 14C-darifenacin solution to healthy volunteers, approximately 60% of the radioactivity was recovered in the urine and 40% in the feces. Only a small percentage of the excreted dose was unchanged darifenacin (3%). Estimated darifenacin clearance is 40 L/h for EMs and 32 L/h for PMs. The elimination half-life of darifenacin following chronic dosing is approximately 13 to 19 hours.
Drug-Drug Interactions
Effects of Other Drugs on Darifenacin
Darifenacin metabolism is primarily mediated by the cytochrome P450 enzymes CYP2D6 and CYP3A4. Therefore, inducers of CYP3A4 or inhibitors of either of these enzymes may alter darifenacin pharmacokinetics [see Drug Interactions (7)].
CYP3A4 Inhibitors: In a drug interaction study, when a 7.5 mg once daily dose of Enablex was given to steady-state and co-administered with the potent CYP3A4 inhibitor ketoconazole 400 mg, mean darifenacin Cmax increased to 11.2 ng/mL for EMs (n=10) and 55.4 ng/mL for one PM subject (n=1). Mean AUC increased to 143 and 939 ng•h/mL for EMs and for one PM subject, respectively. When a 15 mg daily dose of Enablex was given with ketoconazole, mean darifenacin Cmax increased to 67.6 ng/mL and 58.9 ng/mL for EMs (n=3) and one PM subject (n=1), respectively. Mean AUC increased to 1110 and 931 ng•h/mL for EMs and for one PM subject, respectively [see Dosage and Administration (2) and Drug Interactions (7.1)].
The mean Cmax and AUC of darifenacin following 30 mg once daily dosing at steady-state were 128% and 95% higher, respectively, in the presence of a moderate CYP3A4 inhibitor, erythromycin. Co-administration of fluconazole, a moderate CYP3A4 inhibitor and darifenacin 30 mg once daily at steady-state increased darifenacin Cmax and AUC by 88% and 84%, respectively [see Drug Interactions (7.1)].
The mean Cmax and AUC of darifenacin following 30 mg once daily at steady-state were 42% and 34% higher, respectively, in the presence of cimetidine, a mixed CYP P450 enzyme inhibitor.
CYP2D6 Inhibitors: Darifenacin exposure following 30 mg once daily at steady-state was 33% higher in the presence of the potent CYP2D6 inhibitor paroxetine 20 mg [see Drug Interactions (7.2)].
Effects of Darifenacin on Other Drugs
In Vitro Studies: Based on in vitro human microsomal studies, Enablex is not expected to inhibit CYP1A2 or CYP2C9 at clinically relevant concentrations.
In Vivo Studies: The potential for clinical doses of Enablex to act as inhibitors of CYP2D6 or CYP3A4 substrates was investigated in specific drug interaction studies.
CYP2D6 Substrates: The mean Cmax and AUC of imipramine, a CYP2D6 substrate, were increased by 57% and 70%, respectively, in the presence of steady-state darifenacin 30 mg once daily. The mean Cmax and AUC of desipramine, the active metabolite of imipramine, were increased by 260% [see Drug Interactions (7.3)].
CYP3A4 Substrates: Darifenacin (30 mg daily) co-administered with a single oral dose of midazolam 7.5 mg resulted in a 17% increase in midazolam exposure.
Combination Oral Contraceptives: Darifenacin (10 mg three times daily) had no effect on the pharmacokinetics of a combination oral contraceptive containing levonorgestrel (0.15 mg) and ethinyl estradiol (0.03 mg).
Warfarin: Darifenacin had no significant effect on prothrombin time when a single dose of warfarin 30 mg was co-administered with darifenacin (30 mg daily) at steady-state [see Drug Interactions (7.6)].
Digoxin: Darifenacin (30 mg daily) co-administered with digoxin (0.25 mg) at steady-state resulted in a 16% increase in digoxin exposure [see Drug Interactions (7.7)].
Pharmacokinetics in Special Populations
Age
A population pharmacokinetic analysis of patient data indicated a trend for clearance of darifenacin to decrease with age (6% per decade relative to a median age of 44). Following administration of Enablex 15 mg once daily, darifenacin exposure at steady-state was approximately 12% to 19% higher in volunteers between 45 and 65 years of age compared to younger volunteers aged 18 to 44 years [see Use in Specific Populations (8.5)].
Pediatric
The pharmacokinetics of Enablex has not been studied in the pediatric population [see Use in Specific Populations (8.4)].
Gender
PK parameters were calculated for 22 male and 25 female healthy volunteers. Darifenacin Cmax and AUC at steady-state were approximately 57% to 79% and 61% to 73% higher in females than in males, respectively [see Use in Specific Populations (8.8)].
Renal Impairment
A study of subjects with varying degrees of renal impairment (creatinine clearance between 10 and 136 mL/min) given Enablex 15 mg once daily to steady-state demonstrated no clear relationship between renal function and darifenacin clearance [see Use in Specific Populations (8.7)].
Hepatic Impairment
Enablex pharmacokinetics were investigated in subjects with mild (Child-Pugh A) or moderate (Child-Pugh B) impairment of hepatic function given Enablex 15 mg once daily to steady-state. Mild hepatic impairment had no effect on the pharmacokinetics of darifenacin. However, protein binding of darifenacin was affected by moderate hepatic impairment. After adjusting for plasma protein binding, unbound darifenacin exposure was estimated to be 4.7-fold higher in subjects with moderate hepatic impairment than subjects with normal hepatic function. Subjects with severe hepatic impairment (Child-Pugh C) have not been studied [see Dosage and Administration (2), Warning and Precautions (5.5) and Use in Specific Population (8.6)].
13.1. Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity studies with darifenacin were conducted in mice and rats. No evidence of drug-related carcinogenicity was revealed in a 24-month study in mice at dietary doses up to 100 mg/kg/day or approximately 32 times the estimated free plasma AUC reached at the maximum recommended human dose (the AUC at the MRHD) of 15 mg and in a 24-month study in rats at doses up to 15 mg/kg/day or up to approximately 12 times the AUC at the MRHD in female rats and approximately eight times the AUC at the MRHD in male rats.
Darifenacin was not genotoxic in the bacterial mutation assay (Ames test), the Chinese hamster ovary assay, the human lymphocyte assay, or the in vivo mouse bone marrow cytogenetics assay.
There was no evidence for effects on fertility in male or female rats treated at oral doses up to approximately 78 times (50 mg/kg/day) the AUC at the MRHD.
14. Clinical Studies
Enablex extended-release tablets were evaluated for the treatment of patients with overactive bladder with symptoms of urgency, urge urinary incontinence, and increased urinary frequency in three randomized, fixed-dose, placebo-controlled, multicenter, double-blind, 12-week studies (Studies 1, 2 and 3) and one randomized, double-blind, placebo-controlled, multicenter, dose-titration study (Study 4). For study eligibility in all four studies, patients with symptoms of overactive bladder for at least six months were required to demonstrate at least eight micturitions and at least one episode of urinary urgency per day, and at least five episodes of urge urinary incontinence per week. The majority of patients were white (94%) and female (84%), with a mean age of 58 years, range 19 to 93 years. Thirty-three percent of patients were greater than or equal to 65 years of age. These characteristics were well balanced across treatment groups. The study population was inclusive of both naïve patients who had not received prior pharmacotherapy for overactive bladder (60%) and those who had (40%).
Table 4 shows the efficacy data collected from 7- or 14-day voiding diaries in the three fixed-dose placebo-controlled studies of 1,059 patients treated with placebo, 7.5 mg or 15 mg once daily Enablex for 12 weeks. A significant decrease in the primary endpoint, change from baseline in average weekly urge urinary incontinence episodes was observed in all three studies. Data is also shown for two secondary endpoints, change from baseline in the average number of micturitions per day (urinary frequency) and change from baseline in the average volume voided per micturition.
Table 4. Difference Between Enablex (7.5 mg, 15 mg) and Placebo for the Week 12 Change from Baseline (Studies 1, 2 and 3):
| Study 1 | Study 2 | Study 3 | ||||||
|---|---|---|---|---|---|---|---|---|
| Enablex 7.5 mg | Enablex 15 mg | Placebo | Enablex 7.5 mg | Enablex 15 mg | Placebo | Enablex 15 mg | Placebo | |
| No. of Patients Entered | 229 | 115 | 164 | 108 | 107 | 109 | 112 | 115 |
| Urge Incontinence Episodes per Week | ||||||||
| Median Baseline | 16.3 | 17.0 | 16.6 | 14.0 | 17.3 | 16.1 | 16.2 | 15.5 |
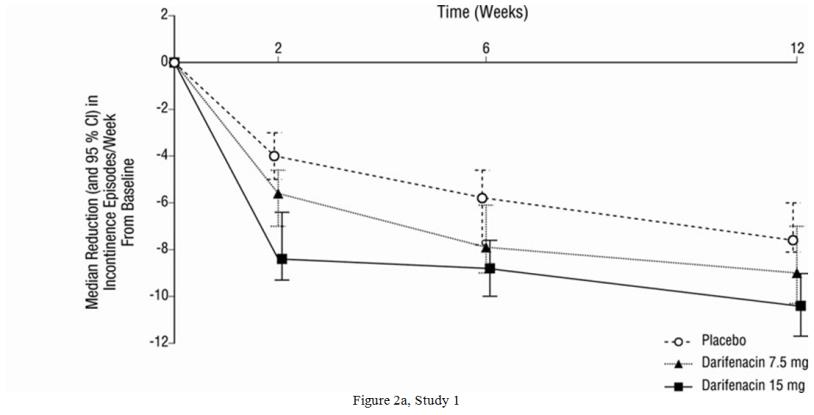
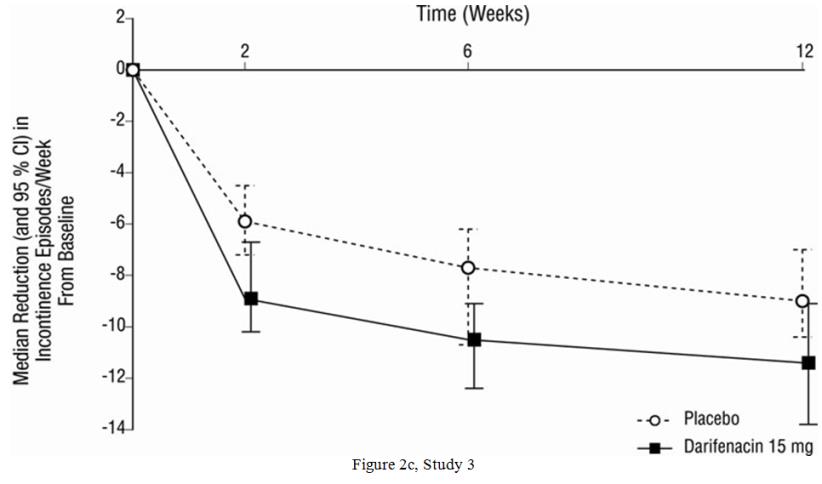
| Median Change from Baseline | -9.0 | -10.4 | -7.6 | -8.1 | -10.4 | -5.9 | -11.4 | -9.0 |
| Median Difference to Placebo | -1.5* | -2.1* | - | -2.8* | -4.3* | - | -2.4* | - |
| Micturitions per Day | ||||||||
| Median Baseline | 10.1 | 10.1 | 10.1 | 10.3 | 11.0 | 10.1 | 10.5 | 10.4 |
| Median Change from Baseline | -1.6 | -1.7 | -0.8 | -1.7 | -1.9 | -1.1 | -1.9 | -1.2 |
| Median Difference to Placebo | -0.8* | -0.9* | - | -0.5 | -0.7* | - | -0.5 | - |
| Volume of Urine Passed per Void (mL) | ||||||||
| Median Baseline | 160.2 | 151.8 | 162.4 | 161.7 | 157.3 | 162.2 | 155.0 | 147.1 |
| Median Change from Baseline | 14.9 | 30.9 | 7.6 | 16.8 | 23.6 | 7.1 | 26.7 | 4.6 |
| Median Difference to Placebo | 9.1* | 20.7* | - | 9.2 | 16.6* | - | 20.1* | - |
* Indicates statistically significant difference versus placebo (p less than 0.05, Wilcoxon rank‑sum test)
Table 5 shows the efficacy data from the dose-titration study in 395 patients who initially received 7.5 mg Enablex or placebo daily with the option to increase to 15 mg Enablex or placebo daily after two weeks.
Table 5. Difference between Enablex (7.5 mg/15 mg) and Placebo for the Week 12 Change from Baseline (Study 4):
| Enablex 7.5 mg/15 mg | Placebo | |
|---|---|---|
| No. of Patients Treated | 268 | 127 |
| Urge Incontinence Episodes per Week | ||
| Median Baseline | 16.0 | 14.0 |
| Median Change from Baseline | -8.2 | -6.0 |
| Median Difference to Placebo | -1.4* | - |
| Micturitions per Day | ||
| Median Baseline | 9.9 | 10.4 |
| Median Change from Baseline | -1.9 | -1.0 |
| Median Difference to Placebo | -0.8* | - |
| Volume of Urine Passed per Void (mL) | ||
| Median Baseline | 173.7 | 177.2 |
| Median Change from Baseline | 18.8 | 6.6 |
| Median Difference to Placebo | 13.3* | - |
* Indicates statistically significant difference versus placebo (p less than 0.05, Wilcoxon rank-sum test)
As seen in Figures 2a, 2b and 2c, reductions in the number of urge incontinence episodes per week were observed within the first two weeks in patients treated with Enablex 7.5 mg and 15 mg once daily compared to placebo. Further, these effects were sustained throughout the 12-week treatment period.
Figures 2a, 2b, 2c. Median Change from Baseline at Weeks 2, 6, 12 for Number of Urge Incontinence Episodes per Week (Studies 1, 2 and 3):
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.