ENTRESTO Film-coated tablet Ref.[10513] Active ingredients: Sacubitril Valsartan Valsartan and Sacubitril
Source: European Medicines Agency (EU) Revision Year: 2023 Publisher: Novartis Europharm Limited, Vista Building, Elm Park, Merrion Road, Dublin 4, Ireland
5.1. Pharmacodynamic properties
Pharmacotherapeutic group: Agents acting on the renin-angiotensin system; angiotensin II receptor blockers (ARBs), other combinations
ATC code: C09DX04
Mechanism of action
Sacubitril/valsartan exhibits the mechanism of action of an angiotensin receptor neprilysin inhibitor by simultaneously inhibiting neprilysin (neutral endopeptidase; NEP) via LBQ657, the active metabolite of the prodrug sacubitril, and by blocking the angiotensin II type-1 (AT1) receptor via valsartan. The complementary cardiovascular benefits of sacubitril/valsartan in heart failure patients are attributed to the enhancement of peptides that are degraded by neprilysin, such as natriuretic peptides (NP), by LBQ657 and the simultaneous inhibition of the effects of angiotensin II by valsartan. NPs exert their effects by activating membrane-bound guanylyl cyclase-coupled receptors, resulting in increased concentrations of the second messenger cyclic guanosine monophosphate (cGMP), which could result in vasodilation, natriuresis and diuresis, increased glomerular filtration rate and renal blood flow, inhibition of renin and aldosterone release, reduction of sympathetic activity, and anti-hypertrophic and anti-fibrotic effects.
Valsartan inhibits detrimental cardiovascular and renal effects of angiotensin II by selectively blocking the AT1 receptor, and also inhibits angiotensin II-dependent aldosterone release. This prevents sustained activation of the renin-angiotensin-aldosterone system that would result in vasoconstriction, renal sodium and fluid retention, activation of cellular growth and proliferation, and subsequent maladaptive cardiovascular remodelling.
Pharmacodynamic effects
The pharmacodynamic effects of sacubitril/valsartan were evaluated after single and multiple dose administrations in healthy subjects and in patients with heart failure, and are consistent with simultaneous neprilysin inhibition and RAAS blockade. In a 7-day valsartan-controlled study in patients with reduced ejection fraction (HFrEF), administration of sacubitril/valsartan resulted in aninitial increase in natriuresis, increased urine cGMP, and decreased plasma levels of mid-regional pro-atrial natriuretic peptide (MR-proANP) and N-terminal prohormone brain natriuretic peptide (NT-proBNP) compared to valsartan. In a 21-day study in HFrEF patients, sacubitril/valsartan significantly increased urine ANP and cGMP and plasma cGMP, and decreased plasma NT-proBNP, aldosterone and endothelin-1 compared to baseline. The AT1-receptor was also blocked as evidenced by increased plasma renin activity and plasma renin concentrations. In the PARADIGM-HF study, sacubitril/valsartan decreased plasma NT-proBNP and increased plasma BNP and urine cGMP compared with enalapril. In the PANORAMA-HF study, a reduction in NT-proBNP was observed at weeks 4 and 12 for sacubitril/valsartan (40.2% and 49.8%) and enalapril (18.0% and 44.9%) compared to baseline. The NT-proBNP levels continued to decrease over the duration of the study with a reduction of 65.1% for sacubitril/valsartan and 61.6% for enalapril at week 52 compared to baseline. BNP is not a suitable biomarker of heart failure in patients treated with sacubitril/valsartan because BNP is a neprilysin substrate (see section 4.4). NT-proBNP is not a neprilysin substrate and is therefore a more suitable biomarker.
In a thorough QTc clinical study in healthy male subjects, single doses of sacubitril/valsartan 194 mg sacubitril/206 mg valsartan and 583 mg sacubitril/617 mg valsartan had no effect on cardiac repolarisation.
Neprilysin is one of multiple enzymes involved in the clearance of amyloid-β (Aβ) from the brain and cerebrospinal fluid (CSF). Administration of sacubitril/valsartan 194 mg sacubitril/206 mg valsartan once daily for two weeks to healthy subjects was associated with an increase in CSF Aβ1-38 compared to placebo; there were no changes in concentrations of CSF Aβ1-40 and 1-42. The clinical relevance of this finding is not known (see section 5.3).
Clinical efficacy and safety
The 24 mg/26 mg, 49 mg/51 mg and 97 mg/103 mg strengths are in some publications referred to as 50, 100 or 200 mg.
PARADIGM-HF
PARADIGM-HF, the pivotal phase 3 study, was a multinational, randomised, double-blind study of 8,442 patients comparing sacubitril/valsartan to enalapril, both given to adult patients with chronic heart failure, NYHA class II-IV and reduced ejection fraction (left ventricular ejection fraction [LVEF] ≤40%, amended later to ≤35%) in addition to other heart failure therapy. The primary endpoint was the composite of cardiovascular (CV) death or hospitalisation for heart failure (HF). Patients with SBP <100 mmHg, severe renal impairment (eGFR <30 ml/min/1.73 m²) and severe hepatic impairment were excluded at screening and therefore not prospectively studied.
Prior to study participation, patients were well treated with standard of care therapy which included ACE inhibitors/ARBs (>99%), beta blockers (94%), mineralocorticoid antagonists (58%) and diuretics (82%). The median follow-up duration was 27 months and patients were treated for up to 4.3 years.
Patients were required to discontinue their existing ACE inhibitor or ARB therapy and enter a sequential single-blind run-in period during which they received treatment with enalapril 10 mg twice daily, followed by single-blind treatment with sacubitril/valsartan 100 mg twice daily, increasing to 200 mg twice daily (see section 4.8 for discontinuations during this period). They were then randomised to the double-blind period of the study, during which they received either sacubitril/valsartan200 mg or enalapril 10 mg twice daily [sacubitril/valsartan (n=4,209); enalapril (n=4,233)].
The mean age of the population studied was 64 years of age and 19% were 75 years of age or older. At randomisation, 70% of patients were NYHA class II, 24% were class III and 0.7% were class IV. The mean LVEF was 29% and there were 963 (11.4%) patients with a baseline LVEF >35% and ≤40%.
In the sacubitril/valsartan group, 76% of patients remained on the target dose of 200 mg twice daily at the end of the study (mean daily dose of 375 mg). In the enalapril group, 75% of patients remained on the target dose of 10 mg twice daily at the end of the study (mean daily dose of 18.9 mg).
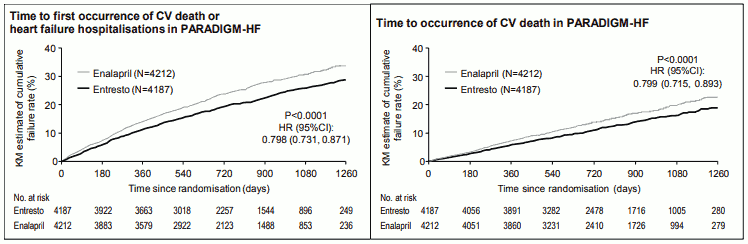
Sacubitril/valsartan was superior to enalapril, reducing the risk of cardiovascular death or heart failure hospitalisations to 21.8% compared to 26.5% for enalapril treated patients. The absolute risk reductions were 4.7% for the composite of the CV death or HF hospitalisation, 3.1% for CV death alone, and 2.8% for first HF hospitalisation alone. The relative risk reduction was 20% versus enalapril (see Table 2). This effect was observed early and was sustained throughout the duration of the study (see Figure 1). Both components contributed to the risk reduction. Sudden death accounted for 45% of cardiovascular deaths and was reduced by 20% in sacubitril/valsartan-treated patients compared to enalapril-treated patients (HR 0.80, p=0.0082). Pump failure accounted for 26% of cardiovascular deaths and was reduced by 21% in sacubitril/valsartan-treated patients compared to enalapril-treated patients (HR 0.79, p=0.0338).
This risk reduction was consistently observed across subgroups including: gender, age, race, geography, NYHA class (II/III), ejection fraction, renal function, history of diabetes or hypertension, prior heart failure therapy, and atrial fibrillation.
Sacubitril/valsartan improved survival with a significant reduction in all-cause mortality of 2.8% (sacubitril/valsartan, 17%, enalapril, 19.8%). The relative risk reduction was 16% compared with enalapril (see Table 3).
Table 3. Treatment effect for the primary composite endpoint, its components and all-cause mortality over a median follow-up of 27 months:
| Sacubitril/valsartan N=4187# n (%) | Enalapril N=4212# n (%) | Hazard ratio (95% CI) | Relative risk reduction | p-value*** | |
|---|---|---|---|---|---|
| Primary composite endpoint of CV death and heart failure hospitalisations* | 914 (21.83) | 1 117 (26.52) | 0.80 (0.73, 0.87) | 20% | 0.0000002 |
| Individual components of the primary composite endpoint | |||||
| CV death** | 558 (13.33) | 693 (16.45) | 0.80 (0.71, 0.89) | 20% | 0.00004 |
| First heart failure hospitalisation | 537 (12.83) | 658 (15.62) | 0.79 (0.71, 0.89) | 21% | 0.00004 |
| Secondary endpoint | |||||
| All-cause mortality | 711 (16.98) | 835 (19.82) | 0.84 (0.76, 0.93) | 16% | 0.0005 |
* The primary endpoint was defined as the time to first event of CV death or hospitalisation for HF.
** CV death includes all patients who died up to the cut-off date irrespective of previous hospitalisation.
*** One-sided p-value
# Full analysis set
Figure 1. Kaplan-Meier curves for the primary composite endpoint and the CV death component:
TITRATION
TITRATION was a 12-week safety and tolerability study in 538 patients with chronic heart failure (NYHA class II–IV) and systolic dysfunction (left ventricular ejection fraction ≤35%) naïve to ACE inhibitor or ARB therapy or on varying doses of ACE inhibitors or ARBs prior to study entry. Patients received a starting dose of sacubitril/valsartan of 50 mg twice daily and were up-titrated to 100 mg twice daily, then to the target dose of 200 mg twice daily, with either a 3-week or a 6-week regimen.
More patients who were naïve to previous ACE inhibitor or ARB therapy or on low-dose therapy (equivalent to <10 mg enalapril/day) were able to achieve and maintain sacubitril/valsartan 200 mg when up-titrated over 6 weeks (84.8%) versus 3 weeks (73.6%). Overall, 76% of patients achieved and maintained the target dose of sacubitril/valsartan 200 mg twice daily without any dose interruption or down-titration over 12 weeks.
Paediatric population
PANORAMA-HF
PANORAMA-HF, a phase 3 study, was a multinational, randomised, double-blind study comparing sacubitril/valsartan and enalapril in 375 paediatric patients aged 1 month to <18 years with heart failure due to systemic left ventricular systolic dysfunction (LVEF ≤45% or fractional shortening ≤22.5%). The primary objective was to determine whether sacubitril/valsartan was superior to enalapril in paediatric HF patients over a 52-week treatment duration based on a global rank endpoint. The global rank primary endpoint was derived by ranking patients (worst-to-best outcome) based on clinical events such as death, initiation of mechanical life support, listing for urgent heart transplant, worsening HF, measures of functional capacity (NYHA/ROSS scores), and patient-reported HF symptoms (Patient Global Impression Scale [PGIS]). Patients with systemic right ventricles or single ventricles and patients with restrictive or hypertrophic cardiomyopathy were excluded from the study. The target maintenance dose of sacubitril/valsartan was 2.3 mg/kg twice daily in paediatric patients aged 1 month to <1 year and 3.1 mg/kg twice daily in patients aged 1 to <18 years with a maximum dose of 200 mg twice daily. The target maintenance dose of enalapril was 0.15 mg/kg twice daily in paediatric patients aged 1 month to <1 year and 0.2 mg/kg twice daily in patients aged 1 to <18 years with a maximum dose of 10 mg twice daily.
In the study, 9 patients were aged 1 month to <1 year, 61 patients were aged 1 year to <2 years, 85 patients were aged 2 to <6 years and 220 patients were aged 6 to <18 years. At baseline, 15.7% of patients were NYHA/ROSS class I, 69.3% were class II, 14.4% were class III and 0.5% were class IV. The mean LVEF was 32%. The most common underlying causes of heart failure were cardiomyopathy related (63.5%). Prior to study participation, patients were treated most commonly with ACE inhibitors/ARBs (93%), beta-blockers (70%), aldosterone antagonists (70%), and diuretics (84%).
The Mann-Whitney Odds of the global rank primary endpoint was 0.907 (95% CI 0.72, 1.14), numerically in favour of sacubitril/valsartan (see Table 4). Sacubitril/valsartan and enalapril showed comparable clinically relevant improvements in the secondary endpoints of NYHA/ROSS class and PGIS score change compared to baseline. At week 52, the NYHA/ROSS functional class changes from baseline were: improved in 37.7% and 34.0%; unchanged in 50.6% and 56.6%; worsened in 11.7% and 9.4% of patients for sacubitril/valsartan and enalapril respectively. Similarly, the PGIS score changes from baseline were: improved in 35.5% and 34.8%; unchanged in 48.0% and 47.5%; worsened in 16.5% and 17.7% of patients for sacubitril/valsartan and enalapril respectively. NT-proBNP was substantially reduced from baseline in both treatment groups. The magnitude of NT-proBNP reduction with Entresto was similar to that observed in adult heart failure patients in PARADIGM-HF. Because sacubitril/valsartan improved outcomes and reduced NT-proBNP in PARADIGM-HF, the reductions in NT-proBNP coupled with the symptomatic and functional improvements from baseline seen in PANORAMA-HF were considered a reasonable basis to infer clinical benefits in paediatric heart failure patients. There were too few patients aged below 1 year to evaluate the efficacy of sacubitril/valsartan in this age group.
Table 4. Treatment effect for the primary global rank endpoint in PANORAMA-HF:
| Sacubitril/valsartan N=187 | Enalapril N=188 | Treatment effect | |
|---|---|---|---|
| Global rank primary endpoint | Probability of favourable outcome (%)* | Probability of favourable outcome (%)* | Odds** (95% CI) |
| 52.4 | 47.6 | 0.907 (0.72, 1.14) |
* The probability of favourable outcome or Mann-Whitney probability (MWP) for the given treatment was estimated based on percentage of wins in pairwise comparisons of global rank score between sacubitril/valsartan-treated patients versus enalapril-treated patients (each higher score counts as one win and each equal score counts as half a win).
** Mann-Whitney Odds was calculated as the estimated MWP for enalapril divided by the estimated MWP for sacubitril/valsartan, with odds <1 in favour of sacubitril/valsartan and >1 in favour of enalapril.
5.2. Pharmacokinetic properties
The valsartan contained within sacubitril/valsartan is more bioavailable than the valsartan in other marketed tablet formulations; 26 mg, 51 mg, and 103 mg of valsartan in sacubitril/valsartan is equivalent to 40 mg, 80 mg and 160 mg of valsartan in other marketed tablet formulations, respectively.
Adult population
Absorption
Following oral administration, sacubitril/valsartan dissociates into valsartan and the prodrug sacubitril. Sacubitril is further metabolised to the active metabolite LBQ657. These reach peak plasma concentrations in 2 hours, 1 hour, and 2 hours, respectively. The oral absolute bioavailability of sacubitril and valsartan is estimated to be more than 60% and 23%, respectively.
Following twice daily dosing of sacubitril/valsartan, steady-state levels of sacubitril, LBQ657 and valsartan are reached in three days. At steady state, sacubitril and valsartan do not accumulate significantly, while LBQ657 accumulates 1.6-fold. Administration with food has no clinically significant impact on the systemic exposures of sacubitril, LBQ657 and valsartan. Sacubitril/valsartan can be administered with or without food.
Distribution
Sacubitril, LBQ657 and valsartan are highly bound to plasma proteins (94-97%). Based on the comparison of plasma and CSF exposures, LBQ657 crosses the blood brain barrier to a limited extent (0.28%). The average apparent volume of distribution of valsartan and sacubitril were 75 litres to 103 litres, respectively.
Biotransformation
Sacubitril is readily converted to LBQ657 by carboxylesterases 1b and 1c; LBQ657 is not further metabolised to a significant extent. Valsartan is minimally metabolised, as only about 20% of the dose is recovered as metabolites. A hydroxyl metabolite of valsartan has been identified in plasma at low concentrations (<10%).
Since CYP450-enzyme-mediated metabolism of sacubitril and valsartan is minimal, co-administration with medicinal products that impact CYP450 enzymes is not expected to impact the pharmacokinetics.
In vitro metabolism studies indicate that potential for CYP450-based drug interactions is low since there is limited metabolism of sacubitril/valsartan via CYP450 enzymes. Sacubitril/valsartan does not induce or inhibit CYP450 enzymes.
Elimination
Following oral administration, 52-68% of sacubitril (primarily as LBQ657) and ~13% of valsartan and its metabolites are excreted in urine; 37-48% of sacubitril (primarily as LBQ657) and 86% of valsartan and its metabolites are excreted in faeces.
Sacubitril, LBQ657 and valsartan are eliminated from plasma with a mean elimination half-life (T½) of approximately 1.43 hours, 11.48 hours, and 9.90 hours, respectively.
Linearity/non-linearity
The pharmacokinetics of sacubitril, LBQ657 and valsartan were approximately linear over a sacubitril/valsartan dose range of 24 mg sacubitril/26 mg valsartan to 97 mg sacubitril/103 mg valsartan.
Special populations
Elderly
LBQ657 and valsartan exposure are increased in subjects over 65 years of age by 42% and 30%, respectively, compared to younger subjects.
Renal impairment
A correlation was observed between renal function and systemic exposure to LBQ657 in patients with mild to severe renal impairment. The exposure of LBQ657 in patients with moderate (30 ml/min/1.73 m² ≤ eGFR <60 ml/min/1.73 m²) and severe renal impairment (15 ml/min/1.73 m² ≤ eGFR <30 ml/min/1.73 m²) was 1.4-fold and 2.2-fold higher compared to patients with mild renal impairment (60 ml/min/1.73 m² ≤ eGFR <90 ml/min/1.73 m²), the largest group of patients enrolled in PARADIGM-HF. The exposure of valsartan was similar in patients with moderate and severe renal impairment compared to patients with mild renal impairment. No studies have been performed in patients undergoing dialysis. However, LBQ657 and valsartan are highly bound to plasma protein and therefore unlikely to be effectively removed by dialysis.
Hepatic impairment
In patients with mild to moderate hepatic impairment, the exposures of sacubitril increased by 1.5- and 3.4- fold, LBQ657 increased by 1.5- and 1.9-fold, and valsartan increased by 1.2-fold and 2.1-fold, respectively, compared to matching healthy subjects. However, in patients with mild to moderate hepatic impairment, the exposures of free concentrations of LBQ657 increased by 1.47- and 3.08-fold, respectively, and the exposures of free concentrations of valsartan increased by 1.09-fold and 2.20-fold, respectively, compared to matching healthy subjects. Sacubitril/valsartan has not been studied in patients with severe hepatic impairment, biliary cirrhosis or cholestasis (see sections 4.3 and 4.4).
Effect of gender
The pharmacokinetics of sacubitril/valsartan (sacubitril, LBQ657 and valsartan) are similar between male and female subjects.
Paediatric population
The pharmacokinetics of sacubitril/valsartan were evaluated in paediatric heart failure patients aged 1 month to <1 year and 1 year to <18 years and indicated that the pharmacokinetic profile of sacubitril/valsartan in paediatric and adult patients is similar.
5.3. Preclinical safety data
Non-clinical data (including studies with sacubitril and valsartan components and/or sacubitril/valsartan) reveal no special hazard for humans based on conventional studies of safety pharmacology, repeated dose toxicity, genotoxicity, carcinogenic potential and fertility.
Fertility, reproduction and development
Sacubitril/valsartan treatment during organogenesis resulted in increased embryofoetal lethality in rats at doses ≥49 mg sacubitril/51 mg valsartan/kg/day (≤0.72-fold the maximum recommended human dose [MRHD] on the basis of AUC) and rabbits at doses ≥4.9 mg sacubitril/5.1 mg valsartan/kg/day (2-fold and 0.03-fold the MRHD on the basis of valsartan and LBQ657 AUC, respectively). It is teratogenic based on a low incidence of foetal hydrocephaly, associated with maternally toxic doses, which was observed in rabbits at a sacubitril/valsartan dose of ≥4.9 mg sacubitril/5.1 mg valsartan/kg/day. Cardiovascular abnormalities (mainly cardiomegaly) were observed in rabbit foetuses at a maternally non-toxic dose (1.46 mg sacubitril/1.54 mg valsartan/kg/day). A slight increase in two foetal skeletal variations (misshapen sternebra, sternebra bipartite ossification) was observed in rabbits at a sacubitril/valsartan dose of 4.9 mg sacubitril/5.1 mg valsartan/kg/day. The adverse embryofoetal effects of sacubitril/valsartan are attributed to the angiotensin receptor antagonist activity (see section 4.6).
Sacubitril treatment during organogenesis resulted in embryo-foetal lethality and embryo-foetal toxicity (decreased foetal body weights and skeletal malformations) in rabbits at doses associated with maternal toxicity (500 mg/kg/day; 5.7-fold the MRHD on the basis of LBQ657 AUC). A slight generalised delay in ossification was observed at doses of >50 mg/kg/day. This finding is not considered adverse. No evidence of embryo-foetal toxicity or teratogenicity was observed in rats treated with sacubitril. The embryo-foetal no-observed adverse effect level (NOAEL) for sacubitril was at least 750 mg/kg/day in rats and 200 mg/kg/day in rabbits (2.2-fold the MRHD on the basis of LBQ657 AUC).
Pre- and postnatal development studies in rats conducted with sacubitril at high doses up to 750 mg/kg/day (2.2-fold the MRHD on the basis of AUC) and valsartan at doses up to 600 mg/kg/day (0.86-fold the MRHD on the basis of AUC) indicate that treatment with sacubitril/valsartan during organogenesis, gestation and lactation may affect pup development and survival.
Other preclinical findings
Sacubitril/valsartan
The effects of sacubitril/valsartan on amyloid-β concentrations in CSF and brain tissue were assessed in young (2-4 years old) cynomolgus monkeys treated with sacubitril/valsartan (24 mg sacubitril/26 mg valsartan/kg/day) for two weeks. In this study CSF Aβ clearance in cynomolgus monkeys was reduced, increasing CSF Aβ1-40, 1-42 and 1-38 levels; there was no corresponding increase in Aβ levels in the brain. Increases in CSF Aβ1-40 and 1-42 were not observed in a two-week healthy volunteer study in humans (see section 5.1). Additionally, in a toxicology study in cynomolgus monkeys treated with sacubitril/valsartan at 146 mg sacubitril/154 mg valsartan/kg/day for 39 weeks, there was no evidence for the presence of amyloid plaques in the brain. Amyloid content was not, however, measured quantitatively in this study.
Sacubitril
In juvenile rats treated with sacubitril (postnatal days 7 to 70), there was a reduction in age-related bone mass development and bone elongation at approximately 2-fold the AUC exposure to the active metabolite of sacubitril, LBQ657, based on sacubitril/valsartan paediatric clinical dose of 3.1 mg/kg twice daily. The mechanism for these findings in juvenile rats, and consequently the relevance to the human paediatric population, is unknown. A study in adult rats showed only a minimal transient inhibitory effect on bone mineral density but not on any other parameters relevant for bone growth, suggesting no relevant effect of sacubitril on bone in adult patient populations under normal conditions. However, a mild transient interference of sacubitril with the early phase of fracture healing in adults cannot be excluded. Clinical data in paediatric patients (PANORAMA-HF study) did not show evidence that sacubitril/valsartan has an impact on body weight, height, head circumference and fracture rate. Bone density was not measured in the study. However, long-term paediatric data on (bone)growth and fracture rates are not available.
Valsartan
In juvenile rats treated with valsartan (postnatal days 7 to 70), doses as low as 1 mg/kg/day produced persistent irreversible kidney changes consisting of tubular nephropathy (sometimes accompanied by tubular epithelial necrosis) and pelvic dilatation. These kidney changes represent an expected exaggerated pharmacological effect of angiotensin converting enzyme inhibitors and angiotensin II type 1 blockers; such effects are observed if rats are treated during the first 13 days of life. This period coincides with 36 weeks of gestation in humans, which could occasionally extend up to 44 weeks after conception in humans. Functional renal maturation is an ongoing process within the first year of life in humans. Consequently, a clinical relevance in paediatric patients less than 1 year of age cannot be excluded, while preclinical data do not indicate a safety concern for paediatric patients older than 1 year.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.