EPIPEN Solution for injection Ref.[50954] Active ingredients: Epinephrine
Source: FDA, National Drug Code (US) Revision Year: 2023
Product description
EpiPen (epinephrine injection, USP) 0.3 mg and EpiPen Jr (epinephrine injection, USP) 0.15 mg are single-dose auto-injectors and combination products containing drug and device components.
Each EpiPen Auto-Injector, 0.3 mg delivers a single dose of 0.3 mg epinephrine from epinephrine injection, USP 0.3 mg/0.3 mL in a sterile solution.
Each EpiPen Jr Auto-Injector, 0.15 mg delivers a single dose of 0.15 mg epinephrine from epinephrine injection, USP 0.15 mg/0.3 mL in a sterile solution.
Each 0.3 mL in the EpiPen Auto-Injector contains 0.3 mg epinephrine, 1.8 mg sodium chloride, 0.5 mg sodium metabisulfite, hydrochloric acid to adjust pH, and Water for Injection. The pH range is 2.2-5.0.
Each 0.3 mL in the EpiPen Jr Auto-Injector contains 0.15 mg epinephrine, 1.8 mg sodium chloride, 0.5 mg sodium metabisulfite, hydrochloric acid to adjust pH, and Water for Injection. The pH range is 2.2-5.0.
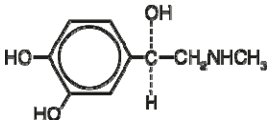
Epinephrine is a sympathomimetic catecholamine. Chemically, epinephrine is (-)3,4 Dihydroxy-α-[(methylamino)methyl]benzyl alcohol with the following structure:
Epinephrine solution deteriorates rapidly on exposure to air or light, turning pink from oxidation to adrenochrome and brown from the formation of melanin. Replace EpiPen and EpiPen Jr if the epinephrine solution appears discolored (pinkish or brown color), cloudy, or contains particles.
Thoroughly review the patient instructions and operation of EpiPen or EpiPen Jr with patients and caregivers prior to use [see Patient Counseling Information (17)]].
| Dosage Forms and Strengths |
|---|
|
| How Supplied |
|---|
EpiPen 2-Pak and EpiPen Jr 2-Pak also include an S-clip to clip two carrier tubes together. Manufactured for Mylan Specialty L.P., Morgantown, WV 26505, U.S.A. by Meridian Medical Technologies, LLC, St. Louis, MO 63146, U.S.A. |
Drugs
| Drug | Countries | |
|---|---|---|
| EPIPEN | Austria, Australia, Canada, Estonia, Finland, France, Croatia, Ireland, Israel, Japan, Lithuania, Malta, Netherlands, New Zealand, Poland, Romania, Singapore, United Kingdom, United States, South Africa |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.