ESTRADERM Transdermal patch Ref.[27763] Active ingredients: Estradiol
Source: Medicines & Healthcare Products Regulatory Agency (GB) Revision Year: 2020 Publisher: Norgine Pharmaceuticals Limited, Norgine House, Widewater Place, Moorhall Road, Harefield, Uxbridge, UB9 6NS, UK
Product name and form
Estraderm MX 25.
| Pharmaceutical Form | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
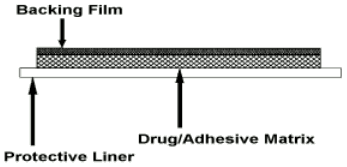
Estraderm MX is a square-shaped, self-adhesive, transparent, transdermal patch for application to the skin surface. Each patch comprises an impermeable polyester backing film, an adhesive matrix containing oestradiol and an oversized protective liner which is removed prior to application of the patch to the skin. Estraderm MX releases oestradiol into the circulation via intact skin at a low rate for up to 4 days. Cross section:
|
Qualitative and quantitative composition
The active ingredient is estra-1, 3,5(10)-triene-3,17β-diol (oestradiol hemihydrate).
Patches contain 0.75 mg active substance corresponding to a surface area of 11cm².
For a full list of excipients, see section 6.1.
| Active Ingredient |
|---|
|
Estradiol, is chemically and biologically identical to endogenous human estradiol. It substitutes for the loss of oestrogen production in menopausal women, and alleviates menopausal symptoms. Oestrogens prevent bone loss following menopause or ovariectomy. |
| List of Excipients |
|---|
|
Acrylate, methacrylate, isopropyl palmitate, polyethylene terephthalate, ethylenevinylacetate copolymer, silicone coating (on the inner side of the protective release liner which is removed before patch application). |
Pack sizes and marketing
Each system is individually heat sealed in a paper/aluminium/polyethylene foil pouch. Eight or twenty four Estraderm MX pouches are placed in an appropriately sized carton which comprises the finished product (one or three month’s treatment respectively).
Marketing authorization holder
Norgine Pharmaceuticals Limited, Norgine House, Widewater Place, Moorhall Road, Harefield, Uxbridge, UB9 6NS, UK
Marketing authorization dates and numbers
Estraderm MX 25: PL 20011/0064
12 September 1997 / 10 February 2009
Drugs
| Drug | Countries | |
|---|---|---|
| ESTRADERM | Australia, Spain, Italy, Poland, United Kingdom, South Africa |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.