ESTRADERM Transdermal patch Ref.[27763] Active ingredients: Estradiol
Source: Medicines & Healthcare Products Regulatory Agency (GB) Revision Year: 2020 Publisher: Norgine Pharmaceuticals Limited, Norgine House, Widewater Place, Moorhall Road, Harefield, Uxbridge, UB9 6NS, UK
4.1. Therapeutic indications
- Hormone replacement therapy (HRT) for oestrogen deficiency symptoms in postmenopausal women[at least 6 months since last menses].
- Prevention of osteoporosis in postmenopausal women at high risk of future fractures who are intolerant of, or contraindicated for, other medicinal products approved for the prevention of osteoporosis (see section 4.4).
The experience treating women older than 65 years is limited.
4.2. Posology and method of administration
Estraderm MX 25 is an oestrogen only patch.
In women with an intact uterus oestrogen should be supplemented by sequential administration of a progestagen (e.g. medroxyprogesterone acetate 10mg, norethisterone 5mg, norethisterone acetate 1-5mg or dydrogesterone 20mg per day) to be taken at least on the last 12 days of each 4-week treatment cycle. Withdrawal bleeding usually occurs following 12 days or more of progesterone administration. Unless there is a previous diagnosis of endometriosis, it is not recommended to add a progestagen in hysterectomised women.
Dosage
Adults and Elderly
Menopausal symptoms: For initiation and continuation of treatment of postmenopausal symptoms, the lowest effective dose for the shortest duration should be used (see also section 4.4). Depending on the clinical response the dose can then be adjusted to the patient's individual needs. If, after three months, there is insufficient response in the form of alleviated symptoms, the dose can be increased.
A maximum dose of 100 micrograms per day should not be exceeded.
Effects usually of estrogenic origin e.g. breast discomfort, water retention or bloating are often observed at the start of treatment, especially in patients receiving hormone replacement therapy for the first time. However, if symptoms persist for more than six weeks the dose should be reduced.
General instructions
Estraderm MX is administered as a continuous sequential treatment (uninterrupted application twice weekly).
For most postmenopausal women not taking HRT Estraderm MX therapy may be started at any convenient time. However, for women with an intact uterus who are still menstruating regularly, commencement within 5 days of the onset of bleeding is recommended.
In women with an intact uterus transferring from a continuous sequential HRT regimen, treatment should begin the day following completion of the prior regimen.
In women transferring from a continuous-combined HRT regimen, or hysterectomised women transferring from other oestrogen-only HRT treatment, treatment may be started on any convenient day.
Administration
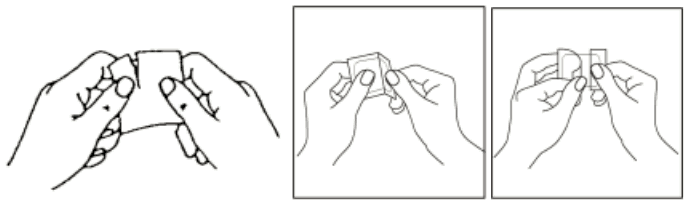
Estraderm MX should be applied immediately after removal of the protective liner (see Figs.), to an area of clean, dry, and intact skin on the trunk below the waistline. The site chosen should be one at which little wrinkling of skin occurs during movement of the body, e.g. buttock. Estraderm MX should never be applied to, or near the breasts.
Estraderm MX should be applied twice weekly on a continuous basis, each used patch being removed after 3-4 days and a fresh system applied to a slightly different site.
If a woman has forgotten to apply a patch, she should apply a new patch as soon as possible. The subsequent patch should be applied according to the original treatment schedule. The interruption of treatment might increase the likelihood of recurrence of symptoms and include breakthrough spotting and bleeding.
In the event that a patch should fall off a new patch may be applied. The original treatment schedule should be continued.
The patch should not be exposed to sunlight.
Special populations
Patients with renal and/or hepatic impairment
No studies were performed in patients with renal and hepatic impairment.
All oestrogen preparations are contraindicated in patients with severe hepatic impairment (see section 4.3 contra-indications).
Children
Estraderm MX is not indicated for use in children.
4.9. Overdose
This is not likely due to the mode of administration.
Signs and Symptoms
Signs of acute oestrogen overdosage may be either one of, or a combination of, breast discomfort, fluid retention and bloating or nausea.
Treatment
Overdosage can if necessary be reversed by removal of the patch(es).
6.3. Shelf life
2 years.
6.4. Special precautions for storage
Store below 25°C.
Keep out of the reach of children both before and after use.
6.5. Nature and contents of container
Each system is individually heat sealed in a paper/aluminium/polyethylene foil pouch. Eight or twenty four Estraderm MX pouches are placed in an appropriately sized carton which comprises the finished product (one or three month's treatment respectively).
6.6. Special precautions for disposal and other handling
See Section 4.2. Exposure of Estraderm MX patches to ultra-violet light results in degradation of oestradiol. Patches should not be exposed to sunlight. They should be applied immediately after removal from the pouch to skin sites covered by clothing.
After use, the Estraderm MX patch should be folded (adhesive surfaces pressed together) and discarded in such a way as to keep them out of the reach and sight of children.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.