FASLODEX Solution for injection Ref.[8323] Active ingredients: Fulvestrant
Source: European Medicines Agency (EU) Revision Year: 2020 Publisher: AstraZeneca AB, SE-151 85 Södertälje, Sweden
Contraindications
- Hypersensitivity to the active substance or to any of the excipients listed in section 6.1.
- Pregnancy and lactation (see section 4.6).
- Severe hepatic impairment (see sections 4.4 and 5.2).
Special warnings and precautions for use
Faslodex should be used with caution in patients with mild to moderate hepatic impairment (see sections 4.2, 4.3 and 5.2).
Faslodex should be used with caution in patients with severe renal impairment (creatinine clearance less than 30 ml/min).
Due to the intramuscular route of administration, Faslodex should be used with caution if treating patients with bleeding diatheses, thrombocytopenia or those taking anticoagulant treatment.
Thromboembolic events are commonly observed in women with advanced breast cancer and have been observed in clinical studies with Faslodex (see section 4.8). This should be taken into consideration when prescribing Faslodex to patients at risk.
Injection site related events including sciatica, neuralgia, neuropathic pain, and peripheral neuropathy have been reported with Faslodex injection. Caution should be taken while administering Faslodex at the dorsogluteal injection site due to the proximity of the underlying sciatic nerve (see sections 4.2 and 4.8).
There are no long-term data on the effect of fulvestrant on bone. Due to the mechanism of action of fulvestrant, there is a potential risk of osteoporosis.
The efficacy and safety of Faslodex (either as monotherapy or in combination with palbociclib) have not been studied in patients with critical visceral disease.
When Faslodex is combined with palbociclib, please also refer to the Summary of Product Characteristics of palbociclib.
Interference with estradiol antibody assays
Due to the structural similarity of fulvestrant and estradiol, fulvestrant may interfere with antibody based-estradiol assays and may result in falsely increased levels of estradiol.
Ethanol
Faslodex contains 10% w/v ethanol (alcohol) as an excipient, i.e. up to 500 mg per injection, equivalent to 10 ml beer or 4 ml wine. This may be harmful for those suffering from alcoholism and should be taken into account in high risk groups such as patients with liver disease and epilepsy.
Benzyl alcohol
Faslodex contains benzyl alcohol as an excipient which may cause allergic reactions.
Paediatric population
Faslodex is not recommended for use in children and adolescents as safety and efficacy have not been established in this group of patients (see section 5.1).
Interaction with other medicinal products and other forms of interaction
A clinical interaction study with midazolam (substrate of CYP3A4) demonstrated that fulvestrant does not inhibit CYP3A4. Clinical interaction studies with rifampicin (inducer of CYP3A4) and ketoconazole (inhibitor of CYP3A4) showed no clinically relevant change in fulvestrant clearance. Dose adjustment is therefore not necessary in patients who are receiving fulvestrant and CYP3A4 inhibitors or inducers concomitantly.
Fertility, pregnancy and lactation
Women of childbearing potential
Patients of childbearing potential should use effective contraception during treatment with Faslodex and for 2 years after the last dose.
Pregnancy
Faslodex is contraindicated in pregnancy (see section 4.3). Fulvestrant has been shown to cross the placenta after single intramuscular doses in rat and rabbit. Studies in animals have shown reproductive toxicity including an increased incidence of foetal abnormalities and deaths (see section 5.3). If pregnancy occurs while taking Faslodex, the patient must be informed of the potential hazard to the foetus and potential risk for loss of pregnancy.
Breast-feeding
Breast-feeding must be discontinued during treatment with Faslodex. Fulvestrant is excreted in milk in lactating rats. It is not known whether fulvestrant is excreted in human milk. Considering the potential for serious adverse reactions due to fulvestrant in breast-fed infants, use during lactation is contraindicated (see section 4.3).
Fertility
The effects of Faslodex on fertility in humans has not been studied.
Effects on ability to drive and use machines
Faslodex has no or negligible influence on the ability to drive or use machines. However, since asthenia has been reported very commonly with Faslodex, caution should be observed by those patients who experience this adverse reaction when driving or operating machinery.
Undesirable effects
Summary of the safety profile
Monotherapy
This section provides information based on all adverse reactions from clinical studies, post-marketing studies or spontaneous reports. In the pooled dataset of fulvestrant monotherapy, the most frequently reported adverse reactions were injection site reactions, asthenia, nausea, and increased hepatic enzymes (ALT, AST, ALP).
In Table 1, the following frequency categories for adverse drug reactions (ADRs) were calculated based on the Faslodex 500 mg treatment group in pooled safety analyses of studies that compared Faslodex 500 mg with Faslodex 250 mg [CONFIRM (Study D6997C00002), FINDER 1 (Study D6997C00004), FINDER 2 (Study D6997C00006), and NEWEST (Study D6997C00003) studies], or from FALCON (Study D699BC00001) alone that compared Faslodex 500 mg with anastrozole 1 mg. Where frequencies differ between the pooled safety analysis and FALCON, the highest frequency is presented. The frequencies in Table 1 were based on all reported adverse drug reactions, regardless of the investigator assessment of causality. The median duration of fulvestrant 500 mg treatment across the pooled dataset (including the studies mentioned above plus FALCON) was 6.5 months.
Tabulated list of adverse reactions
Adverse reactions listed below are classified according to frequency and System Organ Class (SOC). Frequency groupings are defined according to the following convention: Very common (≥1/10), Common (≥1/100 to <1/10), Uncommon (≥1/1,000 to <1/100). Within each frequency grouping adverse reactions are reported in order of decreasing seriousness.
Infections and infestations
Common: Urinary tract infections
Blood and lymphatic system disorders
Common: Reduced platelet counte
Immune system disorders
Very common: Hypersensitivity reactionse
Uncommon: Anaphylactic reactions
Metabolism and nutrition disorders
Common: Anorexiaa
Nervous system disorders
Common: Headache
Vascular disorders
Very common: Hot flushese
Common: Venous thromboembolisma
Gastrointestinal disorders
Very common: Nausea
Common: Vomiting, diarrhoea
Hepatobiliary disorders
Very common: Elevated hepatic enzymes (ALT, AST, ALP)a
Common: Elevated bilirubina
Uncommon: Hepatic failurec,f, hepatitisf, elevated gamma-GTf
Skin and subcutaneous tissue disorders
Very common: Rashe
Musculoskeletal and connective tissue disorders
Very common: Joint and musculoskeletal paind
Common: Back paina
Reproductive system and breast disorders
Common: Vaginal haemorrhagee
Uncommon: Vaginal moniliasisf, leukorrheaf
General disorders and administration site conditions
Very common: Astheniaa, injection site reactionsb
Common: Neuropathy peripherale, sciaticae
Uncommon: Injection site haemorrhagef, injection site haematomaf, neuralgiac,f
a Includes adverse drug reactions for which the exact contribution of Faslodex cannot be assessed due to the underlying disease.
b The term injection site reactions does not include the terms injection site haemorrhage, injection site haematoma, sciatica, neuralgia and neuropathy peripheral.
c The event was not observed in major clinical studies (CONFIRM, FINDER 1, FINDER 2, NEWEST). The frequency has been calculated using the upper limit of the 95% confidence interval for the point estimate. This is calculated as 3/560 (where 560 is the number of patients in the major clinical studies), which equates to a frequency category of ‘uncommon’.
d Includes: arthralgia, and less frequently musculoskeletal pain, myalgia and pain in extremity.
e Frequency category differs between pooled safety dataset and FALCON.
f ADR was not observed in FALCON.
Description of selected adverse reactions
The descriptions included below are based on the safety analysis set of 228 patients who received at least one (1) dose of fulvestrant and 232 patients who received at least one (1) dose of anastrozole, respectively in the Phase 3 FALCON study.
Joint and musculoskeletal pain
In the FALCON study, the number of patients who reported an adverse reaction of joint and musculoskeletal pain was 65 (31.2%) and 48 (24.1%) for fulvestrant and anastrozole arms, respectively. Of the 65 patients in the Faslodex arm, 40% (26/65) of patients reported joint and musculoskeletal pain within the first month of treatment, and 66.2% (43/65) of patients within the first 3 months of treatment. No patients reported events that were CTCAE Grade ≥3 or that required a dose reduction, dose interruption, or discontinued treatment due to these adverse reactions.
Combination therapy with palbociclib
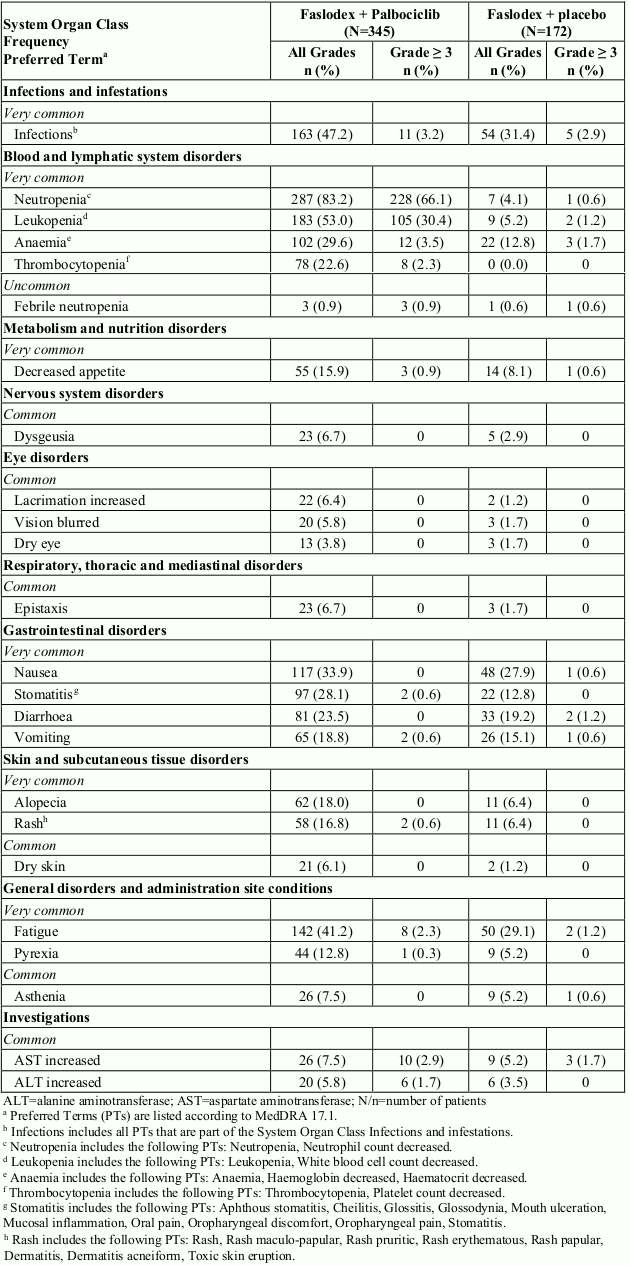
The overall safety profile of fulvestrant when used in combination with palbociclib is based on data from 517 patients with HR-positive, HER2-negative advanced or metastatic breast cancer in the randomised PALOMA3 study (see section 5.1). The most common (≥20%) adverse reactions of any grade reported in patients receiving fulvestrant in combination with palbociclib were neutropenia, leukopenia, infections, fatigue, nausea, anaemia, stomatitis, diarrhoea, and thrombocytopenia. The most common (≥2%) Grade ≥3 adverse reactions were neutropenia, leukopenia, anaemia, infections, AST increased, thrombocytopenia, and fatigue.
Table 2 reports the adverse reactions from PALOMA3. Median duration of exposure to fulvestrant was 11.2 months in the fulvestrant + palbociclib arm and 4.9 months in the fulvestrant + placebo arm. Median duration of exposure to palbociclib in the fulvestrant + palbociclib arm was 10.8 months.
Table 2. Adverse reactions based on PALOMA3 Study (N=517):
Description of selected adverse reactions
Neutropenia
In patients receiving fulvestrant in combination with palbociclib in the PALOMA3 study, neutropenia of any grade was reported in 287 (83.2%) patients, with Grade 3 neutropenia being reported in 191 (55.4%) patients, and Grade 4 neutropenia being reported in 37 (10.7%) patients. In the fulvestrant + placebo arm (n=172), neutropenia of any grade was reported in 7 (4.1%) patients, with Grade 3 neutropenia reported in 1 (0.6%) patient. There were no reports of Grade 4 neutropenia in the fulvestrant + placebo arm.
In patients receiving fulvestrant in combination with palbociclib, the median time to first episode of any grade neutropenia was 15 days (range: 13-317) and the median duration of Grade ≥3 neutropenia was 7 days. Febrile neutropenia has been reported in 0.9% patients receiving fulvestrant in combination with palbociclib.
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the national reporting system listed in Appendix V.
Incompatibilities
In the absence of compatibility studies, this medicinal product must not be mixed with other medicinal products.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.