FASLODEX Solution for injection Ref.[8323] Active ingredients: Fulvestrant
Source: European Medicines Agency (EU) Revision Year: 2020 Publisher: AstraZeneca AB, SE-151 85 Södertälje, Sweden
Therapeutic indications
Faslodex is indicated:
- as monotherapy for the treatment of estrogen receptor positive, locally advanced or metastatic breast cancer in postmenopausal women:
- not previously treated with endocrine therapy, or
- with disease relapse on or after adjuvant antiestrogen therapy, or disease progression on antiestrogen therapy.
- in combination with palbociclib for the treatment of hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative locally advanced or metastatic breast cancer in women who have received prior endocrine therapy (see section 5.1).
In pre- or perimenopausal women, the combination treatment with palbociclib should be combined with a luteinizing hormone releasing hormone (LHRH) agonist.
Posology and method of administration
Posology
Adult females (including Elderly)
The recommended dose is 500 mg at intervals of one month, with an additional 500 mg dose given two weeks after the initial dose.
When Faslodex is used in combination with palbociclib, please also refer to the Summary of Product Characteristics of palbociclib.
Prior to the start of treatment with the combination of Faslodex plus palbociclib, and throughout its duration, pre/perimenopausal women should be treated with LHRH agonists according to local clinical practice.
Special populations
Renal impairment
No dose adjustments are recommended for patients with mild to moderate renal impairment (creatinine clearance ≥30 ml/min). Safety and efficacy have not been evaluated in patients with severe renal impairment (creatinine clearance <30 ml/min), and, therefore, caution is recommended in these patients (see section 4.4).
Hepatic impairment
No dose adjustments are recommended for patients with mild to moderate hepatic impairment. However, as fulvestrant exposure may be increased, Faslodex should be used with caution in these patients. There are no data in patients with severe hepatic impairment (see sections 4.3, 4.4 and 5.2).
Paediatric population
The safety and efficacy of Faslodex in children from birth to 18 years of age have not been established. Currently available data are described in sections 5.1 and 5.2, but no recommendation on a posology can be made.
Method of administration
Faslodex should be administered as two consecutive 5 ml injections by slow intramuscular injection (1-2 minutes/injection), one in each buttock (gluteal area).
Caution should be taken if injecting Faslodex at the dorsogluteal site due to the proximity of the underlying sciatic nerve.
For detailed instructions for administration, see section 6.6.
Overdose
There are isolated reports of overdose with Faslodex in humans. If overdose occurs, symptomatic supportive treatment is recommended. Animal studies suggest that no effects other than those related directly or indirectly to antiestrogenic activity were evident with higher doses of fulvestrant (see section 5.3).
Shelf life
4 years.
Special precautions for storage
Store and transport in a refrigerator (2°C-8°C).
Temperature excursions outside 2°C-8°C should be limited. This includes avoiding storage at temperatures exceeding 30°C, and not exceeding a 28 day period where the average storage temperature for the product is below 25°C (but above 2°C-8°C). After temperature excursions, the product should be returned immediately to the recommended storage conditions (store and transport in a refrigerator 2°C-8°C). Temperature excursions have a cumulative effect on the product quality and the 28 day time period must not be exceeded over the duration of the 4-year shelf life of Faslodex (see section 6.3). Exposure to temperatures below 2°C will not damage the product providing it is not stored below – 20°C.
Store the pre-filled syringe in the original package in order to protect from light.
Nature and contents of container
The pre-filled syringe presentation consists of:
One clear type 1 glass pre-filled syringe with polystyrene plunger rod, fitted with a tamper-evident closure, containing 5 ml Faslodex solution for injection. A safety needle (BD SafetyGlide) for connection to the barrel is also provided.
Or
Two clear type 1 glass pre-filled syringes with polystyrene plunger rod, fitted with a tamper-evident closure, each containing 5 ml Faslodex solution for injection. Safety needles (BD SafetyGlide) for connection to each barrel are also provided.
Not all pack sizes may be marketed.
Special precautions for disposal and other handling
Instructions for administration
Administer the injection according to the local guidelines for performing large volume intramuscular injections.
NOTE: Due to the proximity of the underlying sciatic nerve, caution should be taken if administering Faslodex at the dorsogluteal injection site (see section 4.4).
Warning – Do not autoclave safety needle (BD SafetyGlide Shielding Hypodermic Needle) before use. Hands must remain behind the needle at all times during use and disposal.
For each of the two syringes:
- Remove glass syringe barrel from tray and check that it is not damaged.
- Peel open the safety needle (SafetyGlide) outer packaging.
- Parenteral solutions must be inspected visually for particulate matter and discolouration prior to administration.
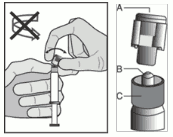
- Hold the syringe upright on the ribbed part ©. With the other hand, take hold of the cap (A) and carefully tilt back and forth until the cap disconnects and can be pulled off, do not twist (see Figure 1).
Figure 1:
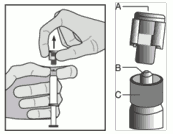
- Remove the cap (A) in a straight upward direction. To maintain sterility do not touch the syringe tip (B) (see Figure 2).
Figure 2:
- Attach the safety needle to the Luer-Lok and twist until firmly seated (see Figure 3).
- Check that the needle is locked to the Luer connector before moving out of the vertical plane.
- Pull shield straight off needle to avoid damaging needle point.
- Transport filled syringe to point of administration.
- Remove needle sheath.
- Expel excess gas from the syringe.
Figure 3:
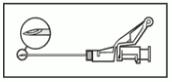
- Administer intramuscularly slowly (1-2 minutes/injection) into the buttock (gluteal area). For user convenience, the needle bevel-up position is oriented to the lever arm (see Figure 4).
Figure 4:
- After injection, immediately apply a single-finger stroke to the activation assisted lever arm to activate the shielding mechanism (see Figure 5).
NOTE: Activate away from self and others. Listen for click
and visually confirm needle tip is fully covered.
Figure 5:
Disposal
Pre-filled syringes are for single use only.
This medicine may pose a risk to the aquatic environment. Any unused medicinal product or waste material should be disposed of in accordance with local requirements (see section 5.3).
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.