FILSPARI Film-coated tablet Ref.[107352] Active ingredients: Sparsentan
Source: FDA, National Drug Code (US) Revision Year: 2023
Product description
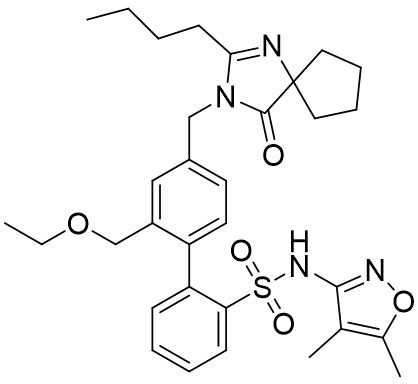
FILSPARI (sparsentan) is an endothelin and angiotensin II receptor antagonist. The chemical name of sparsentan is 2-[4-[(2-butyl-4-oxo-1,3-diazaspiro[4.4]non-1-en-3-yl)methyl]2(ethoxymethyl)phenyl]N(4,5-dimethyl-1,2-oxazol-3-yl)benzenesulfonamide.
Sparsentan is a white to off-white powder, which is practically insoluble in water. Sparsentan has pH-dependent solubility, with intrinsic solubility of 1.48 and 0.055 mg/mL under pH 1.2 and 6.8, respectively. Sparsentan has a molecular weight of 592.76 g/mol, a molecular formula of C32H40N4O5S, and the following structure:
FILSPARI is available as film-coated 200 mg and 400 mg strength immediate release tablets for oral administration.
The inactive ingredients in FILSPARI are colloidal silicon dioxide, lactose anhydrous, magnesium stearate, silicified microcrystalline cellulose, and sodium starch glycolate. Film-coating is composed of macrogol/polyethylene glycol, polyvinyl alcohol-partially hydrolyzed, talc, and titanium dioxide.
| Dosage Forms and Strengths |
|---|
|
FILSPARI is supplied as film-coated, modified oval, white to off-white tablets debossed on one side and plain on the other in the following strengths:
|
| How Supplied |
|---|
|
FILSPARI is supplied in bottles of 30 film-coated tablets.
Distributed by: Travere Therapeutics, Inc., San Diego, CA 92130 Manufactured for: Travere Therapeutics, Inc., San Diego, CA 92130 Manufactured by: Catalent, Kansas City, MO 64137 |
Drugs
| Drug | Countries | |
|---|---|---|
| FILSPARI | Austria, Estonia, France, Italy, Lithuania, United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.