FIRDAPSE Tablet Ref.[9961] Active ingredients: Amifampridine
Source: FDA, National Drug Code (US) Revision Year: 2020
Product description
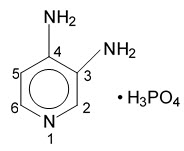
The active ingredient of FIRDAPSE is amifampridine phosphate, which is a voltage-gated potassium channel blocker. Amifampridine phosphate is described chemically as 3,4-diaminopyridine phosphate with a molecular weight of 207.1 and a molecular formula of C5H7N3∙H3PO4.
The structural formula is:
Amifampridine phosphate is a white, crystalline powder that is freely soluble in water, and slightly soluble in solvents ethanol, methanol and acetic acid. A 1% aqueous solution of amifampridine phosphate has a pH of 4.4 at ambient conditions.
Each FIRDAPSE tablet contains 10 mg amifampridine (equivalent to 18.98 mg amifampridine phosphate). The tablet formulation includes the following inactive ingredients: calcium stearate, colloidal silicon dioxide, and microcrystalline cellulose.
FIRDAPSE tablets are intended for oral administration only.
| Dosage Forms and Strengths |
|---|
|
FIRDAPSE tablets contain 10 mg amifampridine and are white to off-white, round, and functionally scored. Each tablet is debossed on the non-scored side with "CATALYST" and on the scored side with "211" above the score and "10" below the score. |
| How Supplied |
|---|
|
FIRDAPSE 10 mg tablets are white to off white, round, and functionally scored. Each tablet is debossed on the non-scored side with "CATALYST" and on the scored side with "211" above the score and "10" below the score. Tablets can be divided in half at the score. FIRDAPSE is supplied as follows: Child Resistant Blister Pack:
Bottles:
|
Drugs
| Drug | Countries | |
|---|---|---|
| FIRDAPSE | Austria, Canada, Estonia, Finland, France, Croatia, Ireland, Israel, Lithuania, Netherlands, Poland, United Kingdom, United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.