FLECTOR Patch Ref.[27463] Active ingredients: Diclofenac
Source: FDA, National Drug Code (US) Revision Year: 2020
12.1. Mechanism of Action
Diclofenac has analgesic, anti-inflammatory, and antipyretic properties.
The mechanism of action of diclofenac, like that of other NSAIDs, is not completely understood but involves inhibition of cyclooxygenase (COX-1 and COX-2).
Diclofenac is a potent inhibitor of prostaglandin synthesis in vitro. Diclofenac concentrations reached during therapy have produced in vivo effects. Prostaglandins sensitize afferent nerves and potentiate the action of bradykinin in inducing pain in animal models. Prostaglandins are mediators of inflammation. Because diclofenac is an inhibitor of prostaglandin synthesis, its mode of action may be due to a decrease of prostaglandins in peripheral tissues.
12.2. Pharmacodynamics
FLECTOR PATCH applied to intact skin provides local analgesia by releasing diclofenac epolamine from the patch into the skin.
12.3. Pharmacokinetics
Absorption
Following a single application of the FLECTOR PATCH on the upper inner arm, peak plasma concentrations of diclofenac (range 0.7–6 ng/mL) were noted between 10–20 hours of application. Plasma concentrations of diclofenac in the range of 1.3–8.8 ng/mL were noted after five days with twice-a-day FLECTOR PATCH application.
Systemic exposure (AUC) and maximum plasma concentrations of diclofenac, after repeated dosing for four days with FLECTOR PATCH, were lower (<1%) than after a single oral 50-mg diclofenac sodium tablet.
The pharmacokinetics of FLECTOR PATCH has been tested in healthy volunteers at rest or undergoing moderate exercise (cycling 20 min/h for 12 h at a mean HR of 100.3 bpm). No clinically relevant differences in systemic absorption were observed, with peak plasma concentrations in the range of 2.2–8.1 ng/mL while resting, and 2.7–7.2 ng/mL during exercise.
Distribution
Diclofenac has a very high affinity (>99%) for human serum albumin. Diclofenac diffuses into and out of the synovial fluid. Diffusion into the joint occurs when plasma levels are higher than those in the synovial fluid, after which the process reverses and synovial fluid levels are higher than plasma levels. It is not known whether diffusion into the joint plays a role in the effectiveness of diclofenac.
Elimination
Metabolism
Five diclofenac metabolites have been identified in human plasma and urine. The metabolites include 4'-hydroxy-, 5-hydroxy-, 3'-hydroxy-, 4',5-dihydroxy- and 3'-hydroxy-4'-methoxy diclofenac. The major diclofenac metabolite, 4'hydroxy-diclofenac, has very weak pharmacologic activity. The formation of 4'-hydroxy diclofenac is primarily mediated by CPY2C9. Both diclofenac and its oxidative metabolites undergo glucuronidation or sulfation followed by biliary excretion. Acylglucuronidation mediated by UGT2B7 and oxidation mediated by CPY2C8 may also play a role in diclofenac metabolism. CYP3A4 is responsible for the formation of minor metabolites, 5-hydroxy and 3'-hydroxy-diclofenac.
Excretion
The plasma elimination half-life of diclofenac after application of FLECTOR PATCH is approximately 12 hours. Diclofenac is eliminated through metabolism and subsequent urinary and biliary excretion of the glucuronide and the sulfate conjugates of the metabolites. Little or no free unchanged diclofenac is excreted in the urine. Approximately 65% of the dose is excreted in the urine and approximately 35% in the bile as conjugates of unchanged diclofenac plus metabolites.
Specific Populations
The pharmacokinetics of FLECTOR PATCH has not been investigated in children, patients with hepatic or renal impairment, or specific racial groups.
Drug Interaction Studies
Aspirin
When NSAIDs were administered with aspirin, the protein binding of NSAIDs were reduced, although the clearance of free NSAID was not altered. The clinical significance of this interaction is not known. See Table 1 for clinically significant drug interactions of NSAIDs with aspirin [see Drug Interactions (7)].
13.1. Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Long-term studies in animals have not been performed to evaluate the carcinogenic potential of either diclofenac epolamine or FLECTOR PATCH.
Mutagenesis
Diclofenac epolamine is not mutagenic in Salmonella typhimurium strains, nor does it induce an increase in metabolic aberrations in cultured human lymphocytes, or the frequency of micronucleated cells in the bone marrow micronucleus test performed in rats.
Impairment of Fertility
Male and female Sprague Dawley rats were administered 1, 3, or 6 mg/kg/day diclofenac epolamine via oral gavage (males treated for 60 days prior to conception and during mating period, females treated for 14 days prior to mating through day 19 of gestation). Diclofenac epolamine treatment with 6 mg/kg/day resulted in increased early resorptions and post-implantation losses; however, no effects on the mating and fertility indices were found. The 6 mg/kg/day dose corresponds to 3 times the maximum recommended daily exposure in humans based on a body surface area comparison.
14. Clinical Studies
14.1 Ankle Sprains
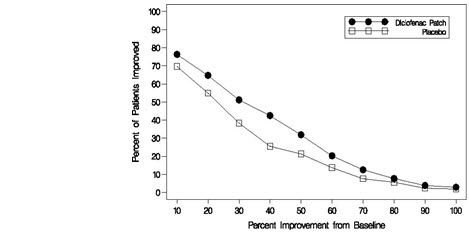
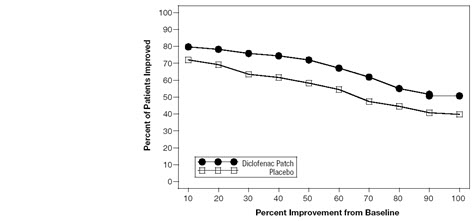
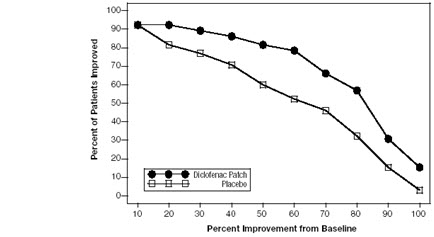
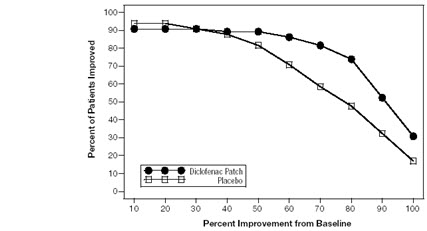
Efficacy of FLECTOR PATCH was demonstrated in two of four studies of patients with minor sprains, strains, and contusions. Patients were randomly assigned to treatment with the FLECTOR PATCH, or a placebo patch identical to the FLECTOR PATCH minus the active ingredient. In the first of these two studies, patients with ankle sprains were treated once daily for a week. In the second study, patients with sprains, strains and contusions were treated twice daily for up to two weeks. Pain was assessed over the period of treatment. Patients treated with the FLECTOR PATCH experienced a greater reduction in pain as compared to patients randomized to placebo patch as evidenced by the responder curves presented below (Figures 1-4).
Figure 1. Patients Achieving Various Levels of Pain Relief at Day 3; 14-Day Study:
Figure 2. Patients Achieving Various Levels of Pain Relief at End of Study; 14-Day Study:
Figure 3. Patients Achieving Various Levels of Pain Relief at Day 3; 7-Day Study:
Figure 4. Patients Achieving Various Levels of Pain Relief at End of Study; 7-Day Study:
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.