GLUCOVANCE Film-coated tablet Ref.[50572] Active ingredients: Glibenclamide Metformin
Source: Web Search Revision Year: 2020 Publisher: Alphapharm Pty Ltd, Level 1, 30 The Bond, 30-34 Hickson Road, Millers Point NSW 2000 www.mylan.com.au
Product name and form
Glucovance.
Metformin hydrochloride and Glibenclamide.
| Pharmaceutical Form |
|---|
|
Oral. Film-coated tablets. Glucovance 250/1.25: Yellow film-coated, capsule-shaped, biconvex tablets, engraved with "250" on one side and "1.25" on the other side. Glucovance 500/2.5: Pale orange film-coated, capsule-shaped, biconvex tablets, engraved with "2.5" on one side. Glucovance 50/5: Yellow film-coated, capsule-shaped, biconvex tablets, engraved with "5" on one side. |
Qualitative and quantitative composition
Glucovance contains metformin hydrochloride and glibenclamide combination and is available in three strength combinations:
Each Glucovance 250/1.25 tablet contains 250 mg metformin hydrochloride and 1.25 mg glibenclamide.
Each Glucovance 500/2.5 tablet contains 500 mg metformin hydrochloride and 2.5 mg glibenclamide.
Each Glucovance 500/5 tablet contains 500 mg metformin hydrochloride and 5 mg glibenclamide.
Glucovance also contains trace amounts of lactose.
For the full list of excipients, see Section 6.1 List of excipients.
Physicochemical properties
Chemical structure
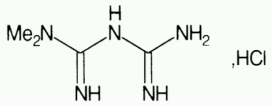
Metformin hydrochloride:
Chemical name: 1,1-dimethylbiguanide hydrochloride
Molecular formula: C4H11N5, HCl
Molecular weight: 165.6
Metformin hydrochloride is a white, crystalline powder, which is odourless or almost odourless and hygroscopic. It is freely soluble in water, slightly soluble in ethanol (96%), and practically insoluble in chloroform and in ether.
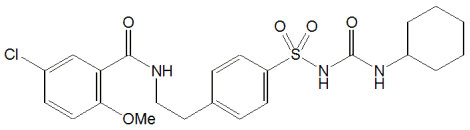
Glibenclamide:
Chemical name: 1-{4-[2-(5-chloro-2-methoxybenzamido)ethyl]benzene-sulphonyl}-3-cyclohexylurea
Molecular formula: C23H28ClN3O5S
Molecular weight: 494
Glibenclamide is a white or almost white, crystalline powder; odourless or almost odourless. It is practically insoluble in water and in ether, slightly soluble in ethanol and methanol and sparingly soluble in chloroform.
CAS number
Metformin hydrochloride: 1115-70-4
Glibenclamide: 10238-21-8
| Active Ingredient |
|---|
|
Glibenclamide, a second-generation, short half-life sulphonylurea, is a hypoglycaemic agent that reduces blood-glucose by stimulating insulin release by the pancreas. Sulphonylureas act on pancreatic beta-cells by inhibiting ATP-sensitive potassium channels. |
|
Metformin is a biguanide with antihyperglycaemic effects, lowering both basal and postprandial plasma glucose. It does not stimulate insulin secretion and therefore does not produce hypoglycaemia. |
| List of Excipients |
|---|
|
Glucovance 250/1.25, Glucovance 500/2.5 and Glucovance 500/5 tablets contain croscarmellose sodium, povidone, magnesium stearate, cellulose – microcrystalline. Glucovance 250/1.25 tablet also contains Opadry II complete film coating system OY-L-22903 Yellow. Glucovance 500/2.5 tablet also contains Opadry II complete film coating system OY-L-24808 Pink Glucovance 500/5 tablet also contains Opadry II complete film coating system 31F22700 Yellow. All three tablets strengths are gluten free. |
Pack sizes and marketing
Glucovance 250/1.25: Blister pack 10s, 30s, 60s, 90s, 120s.
Glucovance 500/2.5: Blister pack 10s, 30s, 60s, 90s, 120s.
Glucovance 500/5: Blister pack 10s, 30s, 60s, 90s, 120s.
Some strengths and/or pack sizes may not be marketed.
Marketing authorization holder
Alphapharm Pty Ltd, Level 1, 30 The Bond, 30-34 Hickson Road, Millers Point NSW 2000
www.mylan.com.au
Marketing authorization dates and numbers
Date of first approval: 9 September 2008
Drugs
| Drug | Countries | |
|---|---|---|
| GLUCOVANCE | Brazil, Ecuador, France, Hong Kong, Mexico, Nigeria, Netherlands, Romania, Singapore, Tunisia, South Africa |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.