IBRANCE Hard capsule Ref.[9143] Active ingredients: Palbociclib
Source: European Medicines Agency (EU) Revision Year: 2023 Publisher: Pfizer Europe MA EEIG, Boulevard de la Plaine 17, 1050, Bruxelles, Belgium
Pharmacodynamic properties
Pharmacotherapeutic group: Antineoplastic agents, protein kinase inhibitors
ATC code: L01EF01
Mechanism of action
Palbociclib is a highly selective, reversible inhibitor of cyclin-dependent kinases (CDK) 4 and 6. Cyclin D1 and CDK4/6 are downstream of multiple signalling pathways which lead to cellular proliferation.
Pharmacodynamic effects
Through inhibition of CDK4/6, palbociclib reduced cellular proliferation by blocking progression of the cell from G1 into S phase of the cell cycle. Testing of palbociclib in a panel of molecularly profiled breast cancer cell lines revealed high activity against luminal breast cancers, particularly ER-positive breast cancers. In the cell lines tested, the loss of retinoblastoma (Rb) was associated with loss of palbociclib activity. However, in a follow-up study with fresh tumour samples, no relation between RB1 expression and tumour response was observed. Similarly, no relation was observed when studying the response to palbociclib in in vivo models with patient-derived xenografts (PDX models). Available clinical data are reported in the clinical efficacy and safety section (see section 5.1).
Cardiac electrophysiology
The effect of palbociclib on the QT interval corrected for heart rate (QTc) interval was evaluated using time matched electrocardiogram (ECG) evaluating the change from baseline and corresponding pharmacokinetic data in 77 patients with advanced breast cancer. Palbociclib did not prolong the QTc to any clinically relevant extent at the recommended dose of 125 mg daily (Schedule 3/1).
Clinical efficacy and safety
Randomised Phase 3 Study PALOMA-2: IBRANCE in combination with letrozole
The efficacy of palbociclib in combination with letrozole versus letrozole plus placebo was evaluated in an international, randomised, double-blind, placebo-controlled, parallel-group, multicentre study conducted in women with ER-positive, HER2-negative locally advanced breast cancer not amenable to resection or radiation therapy with curative intent or metastatic breast cancer who had not received prior systemic treatment for their advanced disease.
A total of 666 postmenopausal women were randomised 2:1 to the palbociclib plus letrozole arm or placebo plus letrozole arm and were stratified by site of disease (visceral versus nonvisceral), disease-free interval from the end of (neo)adjuvant treatment to disease recurrence (de novo metastatic versus 12 months versus >12 months), and by the type of prior (neo)adjuvant anticancer therapies (prior hormonal therapy versus no prior hormonal therapy). Patients with advanced symptomatic, visceral spread, that were at risk of life-threatening complications in the short term (including patients with massive uncontrolled effusions [pleural, pericardial, peritoneal], pulmonary lymphangitis, and over 50% liver involvement), were not eligible for enrolment into the study.
Patients continued to receive assigned treatment until objective disease progression, symptomatic deterioration, unacceptable toxicity, death, or withdrawal of consent, whichever occurred first. Crossover between treatment arms was not allowed.
Patients were well matched for baseline demographics and prognostic characteristics between the palbociclib plus letrozole arm and the placebo plus letrozole arm. The median age of patients enrolled in this study was 62 years (range 28-89), 48.3% of patients had received chemotherapy and 56.3% had received antihormonal therapy in the (neo)adjuvant setting prior to their diagnosis of advanced breast cancer while 37.2% of patients had received no prior systemic therapy in the (neo)adjuvant setting. The majority of patients (97.4%) had metastatic disease at baseline, 23.6% of patients had bone-only disease, and 49.2% of patients had visceral disease.
The primary endpoint of the study was progression-free survival (PFS) evaluated according to Response Evaluation Criteria in Solid Tumours (RECIST) v1.1, as assessed by investigator. Secondary efficacy endpoints included objective response (OR), clinical benefit response (CBR), safety, and change in quality of life (QoL).
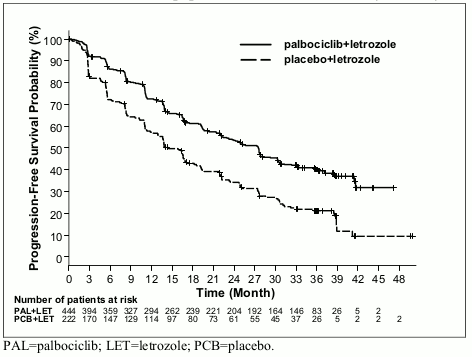
At the data cutoff date of 26-February-2016, the study met its primary objective of improving PFS. The observed hazard ratio (HR) was 0.576 (95% confidence interval [CI]: 0.46, 0.72) in favour of palbociclib plus letrozole, with a stratified log-rank test 1-sided p-value of <0.000001. An updated analysis of the primary and secondary endpoints was performed after an additional 15 months of follow up (data cutoff date: 31-May-2017). A total of 405 PFS events were observed; 245 events (55.2%) in the palbociclib plus letrozole arm and 160 (72.1%) in the comparator arm respectively.
Table 6 shows the efficacy results based on the primary and the updated analyses from the PALOMA-2 study, as assessed by the investigator and by the independent review.
Table 6. PALOMA-2 (intent-to-treat population) - Efficacy results based on primary and updated cutoff dates:
| Primary analysis (26 February 2016 cutoff) | Updated analysis (31 May 2017 cutoff) | |||
|---|---|---|---|---|
| IBRANCE plus letrozole (N=444) | Placebo plus letrozole (N=222) | IBRANCE plus letrozole (N=444) | Placebo plus letrozole (N=222) | |
| Progression-free survival by investigator sssessment | ||||
| Number of events (%) | 194 (43.7) | 137 (61.7) | 245 (55.2) | 160 (72.1) |
| Median PFS [months (95% CI)] | 24.8 (22.1, NE) | 14.5 (12.9, 17.1) | 27.6 (22.4, 30.3) | 14.5 (12.3, 17.1) |
| Hazard ratio [(95% CI) and p-value] | 0.576 (0.463, 0.718), p<0.000001 | 0.563 (0.461, 0.687), p<0.000001 | ||
| Progression-free survival by independent assessment | ||||
| Number of events (%) | 152 (34.2) | 96 (43.2) | 193 (43.5) | 118 (53.2) |
| Median PFS [months (95% CI)] | 30.5 (27.4, NE) | 19.3 (16.4, 30.6) | 35.7 (27.7, 38.9) | 19.5 (16.6, 26.6) |
| Hazard ratio (95% CI) and 1-sided p-value | 0.653 (0.505, 0.844), p=0.000532 | 0.611 (0.485, 0.769), p=0.000012 | ||
| OR* [% (95% CI)] | 46.4 (41.7, 51.2) | 38.3 (31.9, 45.0) | 47.5 (42.8, 52.3) | 38.7 (32.3, 45.5) |
| OR*, measurable disease [% (95% CI)] | 60.7 (55.2, 65.9) | 49.1 (41.4, 56.9) | 62.4 (57.0, 67.6) | 49.7 (42.0, 57.4) |
| CBR* [% (95% CI)] | 85.8 (82.2, 88.9) | 71.2 (64.7, 77.0) | 85.6 (82.0, 88.7) | 71.2 (64.7, 77.0) |
N=number of patients; CI=confidence interval; NE=not estimable; OR=objective response; CBR=clinical benefit response; PFS=progression-free survival.
* Secondary endpoints results are based on confirmed and unconfirmed responses according to RECIST 1.1.
The Kaplan-Meier curves for PFS based on the updated cutoff date of 31 May 2017 are displayed in Figure 1 below.
Figure 1. Kaplan-Meier plot of progression-free survival (investigator assessment, intent-to-treat population) – PALOMA-2 study (31-May-2017):
A series of prespecified subgroup PFS analyses was performed based on prognostic factors and baseline characteristics to investigate the internal consistency of treatment effect. A reduction in the risk of disease progression or death in favour of the palbociclib plus letrozole arm was observed in all individual patient subgroups defined by stratification factors and baseline characteristics in the primary and in the updated analysis.
Based on the 31-May-2017 data cutoff date, this reduction in risk continued to be observed in the following subgroups: (1) patients with either visceral metastases (HR of 0.62 [95% CI: 0.47, 0.81], median progression-free survival [mPFS] 19.3 months versus 12.3 months) or without visceral metastases (HR of 0.50 [95% CI: 0.37, 0.67], mPFS 35.9 months versus 17.0 months) and (2) patients with either bone only disease (HR of 0.41 [95% CI: 0.26, 0.63], mPFS 36.2 months versus 11.2 months) or without bone-only disease (HR of 0.62 [95% CI: 0.50, 0.78], mPFS 24.2 months versus 14.5 months). Similarly, a reduction in the risk of disease progression or death in the palbociclib plus letrozole arm was observed in 512 patients whose tumour tested positive for Rb protein expression by immunohistochemistry (IHC) (HR of 0.543 [95% CI: 0.433, 0.681], mPFS 27.4 months versus 13.7 months). For the 51 patients IHC negative for Rb expression, the difference between treatment arms was not statistically significant (HR of 0.868 [95% CI: 0.424, 1.777], mPFS 23.2 versus 18.5 months) for the palbociclib plus letrozole arm versus the placebo plus letrozole arm, respectively.
Additional efficacy measures (OR and time to response [TTR]) assessed in the sub-groups of patients with or without visceral disease based on the 31-May-2017 updated cutoff date are displayed in Table 7.
Table 7. Efficacy results in patients with visceral or non-visceral disease from PALOMA–2 study (intent-to-treat population; 31-May-2017 cutoff date):
| Visceral disease | Non-visceral disease | |||
|---|---|---|---|---|
| IBRANCE plus letrozole (N=214) | Placebo plus letrozole (N=110) | IBRANCE plus letrozole (N=230) | Placebo plus letrozole (N=112) | |
| OR [% (95% CI)] | 59.8 (52.9, 66.4) | 46.4 (36.8, 56.1) | 36.1 (29.9, 42.7) | 31.3 (22.8, 40.7) |
| TTR, Median [months (range)] | 5.4 (2.0, 30.4) | 5.3 (2.6, 27.9) | 3.0 (2.1, 27.8) | 5.5 (2.6, 22.2) |
N=number of patients; CI=confidence interval; OR=objective response based on confirmed and unconfirmed responses according to RECIST 1.1; TTR=time to first tumour response.
At the time of the updated analyses, the median time from randomisation to second subsequent therapy was 38.8 months in the palbociclib + letrozole arm and 28.8 months in the placebo + letrozole arm, HR 0.73 (95% CI: 0.58, 0.91).
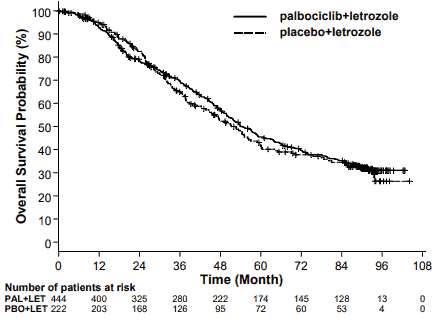
The results from the final OS analysis from the PALOMA-2 study are presented in Table 8. After a median follow-up time of 90 months, the final OS results were not statistically significant. The Kaplan-Meier plot of OS is shown in Figure 2.
Table 8. PALOMA-2 (intent-to-treat population) – Final overall survival results:
| Final Overall Survival (OS) (15 November 2021 Cutoff) | ||
|---|---|---|
| IBRANCE plus letrozole (N=444) | Placebo plus letrozole (N=222) | |
| Number of events (%) | 273 (61.5) | 132 (59.5) |
| Number of subjects remaining in follow-up (%) | 112 (25.2) | 43 (19.4) |
| Median OS (months [95% CI]) | 53.9 (49.8, 60.8) | 51.2 (43.7, 58.9) |
| Hazard ratio (95% CI) and p-value† | 0.956 (0.777, 1.177), p=0.6755†* | |
CI=confidence interval.
* Not statistically significant.
† 2-sided p-value from the log-rank test stratified by disease site (visceral vs. non-visceral) per randomisation.
PAL=palbociclib; LET=letrozole; PBO=placebo.
Randomised Phase 3 Study PALOMA-3: IBRANCE in combination with fulvestrant
The efficacy of palbociclib in combination with fulvestrant versus fulvestrant plus placebo was evaluated in an international, randomised, double-blind, parallel-group, multicentre study conducted in women with HR-positive, HER2-negative locally advanced breast cancer not amenable to resection or radiation therapy with curative intent or metastatic breast cancer, regardless of their menopausal status, whose disease progressed after prior endocrine therapy in the (neo)adjuvant or metastatic setting.
A total of 521 pre/peri- and postmenopausal women who had progressed on or within 12 months from completion of adjuvant endocrine therapy or on or within 1 month from prior endocrine therapy for advanced disease, were randomised 2:1 to palbociclib plus fulvestrant or placebo plus fulvestrant and stratified by documented sensitivity to prior hormonal therapy, menopausal status at study entry (pre/peri- versus postmenopausal), and presence of visceral metastases. Pre/perimenopausal women received the LHRH agonist goserelin. Patients with advanced/metastatic, symptomatic, visceral spread, that were at risk of life-threatening complications in the short term (including patients with massive uncontrolled effusions [pleural, pericardial, peritoneal], pulmonary lymphangitis, and over 50% liver involvement), were not eligible for enrolment into the study.
Patients continued to receive assigned treatment until objective disease progression, symptomatic deterioration, unacceptable toxicity, death, or withdrawal of consent, whichever occurred first. Crossover between treatment arms was not allowed.
Patients were well matched for baseline demographics and prognostic characteristics between the palbociclib plus fulvestrant arm and the placebo plus fulvestrant arm. The median age of patients enrolled in this study was 57 years (range 29, 88). In each treatment arm the majority of patients were White, had documented sensitivity to prior hormonal therapy, and were postmenopausal. Approximately 20% of patients were pre/perimenopausal. All patients had received prior systemic therapy and most patients in each treatment arm had received a previous chemotherapy regimen for their primary diagnosis. More than half (62%) had an ECOG PS of 0, 60% had visceral metastases, and 60% had received more than 1 prior hormonal regimen for their primary diagnosis.
The primary endpoint of the study was investigator-assessed PFS evaluated according to RECIST 1.1. Supportive PFS analyses were based on an Independent Central Radiology Review. Secondary endpoints included OR, CBR, overall survival (OS), safety, and time-to-deterioration (TTD) in pain endpoint.
The study met its primary endpoint of prolonging investigator-assessed PFS at the interim analysis conducted on 82% of the planned PFS events; the results crossed the prespecified Haybittle-Peto efficacy boundary (α=0.00135), demonstrating a statistically significant prolongation in PFS and a clinically meaningful treatment effect. A more mature update of efficacy data is reported in Table 8.
After a median follow-up time of 45 months, the final OS analysis was performed based on 310 events (60% of randomised patients). A 6.9-month difference in median OS in the palbociclib plus fulvestrant arm compared with the placebo plus fulvestrant arm was observed; this result was not statistically significant at the prespecified significance level of 0.0235 (1-sided). In the placebo plus fulvestrant arm, 15.5% of randomised patients received palbociclib and other CDK inhibitors as post progression subsequent treatments.
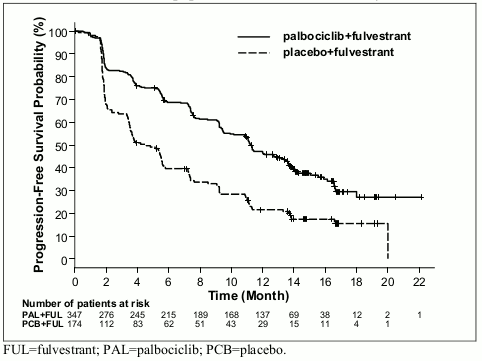
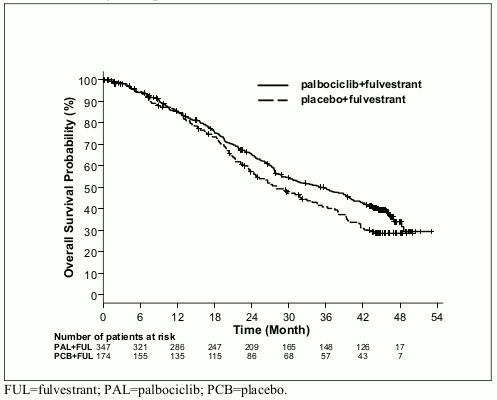
The results from the investigator-assessed PFS and final OS data from PALOMA-3 study are presented in Table 9. The relevant Kaplan-Meier plots are shown in Figures 3 and 4, respectively.
Table 9. Efficacy results – PALOMA-3 study (investigator assessment, intent-to-treat population):
| Updated analysis (23 October 2015 cutoff) | ||
|---|---|---|
| IBRANCE plus fulvestrant (N=347) | Placebo plus fulvestrant (N=174) | |
| Progression-free survival (PFS) | ||
| Number of events (%) | 200 (57.6%) | 133 (76.4%) |
| Median [months (95% CI)] | 11.2 (9.5, 12.9) | 4.6 (3.5, 5.6) |
| Hazard ratio (95% CI) and p-value | 0.497 (0.398, 0.620), p<0.000001 | |
| Secondary efficacy endpoints | ||
| OR [% (95% CI)] | 26.2 (21.7, 31.2) | 13.8 (9.0, 19.8) |
| OR (measurable disease) [% (95% CI)] | 33.7 (28.1, 39.7) | 17.4 (11.5, 24.8) |
| CBR [% (95% CI)] | 68.0 (62.8, 72.9) | 39.7 (32.3, 47.3) |
| Final overall survival (OS) (13 April 2018 cutoff) | ||
| Number of events (%) | 201 (57.9) | 109 (62.6) |
| Median [months (95% CI)] | 34.9 (28.8, 40.0) | 28.0 (23.6, 34.6) |
| Hazard ratio (95% CI) and p-value† | 0.814 (0.644, 1.029) p=0.0429†* | |
CBR=clinical benefit response; CI=confidence interval; N=number of patients; OR=objective response. Secondary endpoint results are based on confirmed and unconfirmed responses according to RECIST 1.1.
* Not statistically significant.
† 1-sided p-value from the log-rank test stratified by the presence of visceral metastases and sensitivity to prior endocrine therapy per randomisation.
Figure 3. Kaplan-Meier plot of progression-free survival (investigator assessment, intent-to-treat population) – PALOMA-3 study (23 October 2015 cutoff):
A reduction in the risk of disease progression or death in the palbociclib plus fulvestrant arm was observed in all individual patient subgroups defined by stratification factors and baseline characteristics. This was evident for pre/perimenopausal women (HR of 0.46 [95% CI: 0.28, 0.75]) and postmenopausal women (HR of 0.52 [95% CI: 0.40, 0.66]) and patients with visceral site of metastatic disease (HR of 0.50 [95% CI: 0.38, 0.65]) and non-visceral site of metastatic disease (HR of 0.48 [95% CI: 0.33, 0.71]). Benefit was also observed regardless of lines of prior therapy in the metastatic setting, whether 0 (HR of 0.59 [95% CI: 0.37, 0.93]), 1 (HR of 0.46 [95% CI: 0.32, 0.64]), 2 (HR of 0.48 [95% CI: 0.30, 0.76]), or ≥3 lines (HR of 0.59 [95% CI: 0.28, 1.22]).
Figure 4. Kaplan-Meier plot of overall survival (intent-to-treat population) – PALOMA-3 study (13 April 2018 cutoff):
Additional efficacy measures (OR and TTR) assessed in the sub-groups of patients with or without visceral disease are displayed in Table 10.
Table 10. Efficacy results in visceral and non-visceral disease from PALOMA–3 study (intent-to-treat population):
| Visceral disease | Non-visceral disease | |||
|---|---|---|---|---|
| IBRANCE plus fulvestrant (N=206) | Placebo plus fulvestrant (N=105) | IBRANCE plus fulvestrant (N=141) | Placebo plus fulvestrant (N=69) | |
| OR [, (95 CI)] | 35.0 (28.5, 41.9) | 13.3 (7.5, 21.4) | 13.5 (8.3, 20.2) | 14.5 (7.2, 25.0) |
| TTR, Median [months (range)] | 3.8 (3.5, 16.7) | 5.4 (3.5, 16.7) | 3.7 (1.9, 13.7) | 3.6 (3.4, 3.7) |
N=number of patients; CI=confidence interval; OR=objective response based on confirmed and unconfirmed responses according to RECIST 1.1; TTR=time to first tumour response.
Patient-reported symptoms were assessed using the European Organisation for Research and Treatment of Cancer (EORTC) quality of life questionnaire (QLQ)-C30 and its Breast Cancer Module (EORTC QLQ-BR23). A total of 335 patients in the palbociclib plus fulvestrant arm and 166 patients in the fulvestrant only arm completed the questionnaire at baseline and at least 1 postbaseline visit.
Time-to-Deterioration was prespecified as time between baseline and first occurrence of ≥10 points increase from baseline in pain symptom scores. Addition of palbociclib to fulvestrant resulted in a symptom benefit by significantly delaying time-to-deterioration in pain symptom compared with placebo plus fulvestrant (median 8.0 months versus 2.8 months; HR of 0.64 [95% CI: 0.49, 0.85]; p<0.001).
The European Medicines Agency has waived the obligation to submit the results of studies with IBRANCE in all subsets of the paediatric population in the treatment of breast carcinoma (see section 4.2 for information on paediatric use).
Pharmacokinetic properties
The pharmacokinetics of palbociclib were characterised in patients with solid tumours including advanced breast cancer and in healthy volunteers.
Absorption
The mean Cmax of palbociclib is generally observed between 6 to 12 hours following oral administration. The mean absolute bioavailability of palbociclib after an oral 125 mg dose is 46%. In the dosing range of 25 mg to 225 mg, the area under the curve (AUC) and Cmax increase proportionally with dose in general. Steady state was achieved within 8 days following repeated once daily dosing. With repeated once daily administration, palbociclib accumulates with a median accumulation ratio of 2.4 (range 1.5-4.2).
Food effect
Palbociclib absorption and exposure were very low in approximately 13% of the population under the fasted condition. Food intake increased the palbociclib exposure in this small subset of the population, but did not alter palbociclib exposure in the rest of the population to a clinically relevant extent. Compared to palbociclib given under overnight fasted conditions, the AUCinf and Cmax of palbociclib increased by 21% and 38% when given with high-fat food, by 12% and 27% when given with low-fat food, and by 13% and 24% when moderate-fat food was given 1 hour before and 2 hours after palbociclib dosing. In addition, food intake significantly reduced the intersubject and intrasubject variability of palbociclib exposure. Based on these results, palbociclib should be taken with food (see section 4.2).
Distribution
Binding of palbociclib to human plasma proteins in vitro was ~85%, with no concentration dependence. The mean fraction unbound (fu) of palbociclib in human plasma in vivo increased incrementally with worsening hepatic function. There was no obvious trend in the mean palbociclib fu in human plasma in vivo with worsening renal function. In vitro, the uptake of palbociclib into human hepatocytes occurred mainly via passive diffusion. Palbociclib is not a substrate of OATP1B1 or OATP1B3.
Biotransformation
In vitro and in vivo studies indicate that palbociclib undergoes extensive hepatic metabolism in humans. Following oral administration of a single 125 mg dose of [14C]palbociclib to humans, the major primary metabolic pathways for palbociclib involved oxidation and sulphonation, with acylation and glucuronidation contributing as minor pathways. Palbociclib was the major circulating drug-derived entity in plasma.
The majority of the material was excreted as metabolites. In faeces, the sulfamic acid conjugate of palbociclib was the major drug-related component, accounting for 25.8% of the administered dose. In vitro studies with human hepatocytes, liver cytosolic and S9 fractions, and recombinant sulphotransferase (SULT) enzymes indicated that CYP3A and SULT2A1 are mainly involved in the metabolism of palbociclib.
Elimination
The geometric mean apparent oral clearance (CL/F) of palbociclib was 63 L/h, and the mean plasma elimination half-life was 28.8 hours in patients with advanced breast cancer. In 6 healthy male subjects given a single oral dose of [14C]palbociclib, a median of 92% of the total administered radioactive dose was recovered in 15 days; faeces (74% of dose) was the major route of excretion, with 17% of the dose recovered in urine. Excretion of unchanged palbociclib in faeces and urine was 2% and 7% of the administered dose, respectively.
In vitro, palbociclib is not an inhibitor of CYP1A2, 2A6, 2B6, 2C8, 2C9, 2C19, and 2D6, and is not an inducer of CYP1A2, 2B6, 2C8, and 3A4 at clinically relevant concentrations.
In vitro evaluations indicate that palbociclib has low potential to inhibit the activities of organic anion transporter (OAT)1, OAT3, organic cation transporter (OCT)2, organic anion transporting polypeptide (OATP)1B1, OATP1B3, and bile salt export pump (BSEP) at clinically relevant concentrations.
Special populations
Age, gender, and body weight
Based on a population pharmacokinetic analysis in 183 patients with cancer (50 male and 133 female patients, age ranging from 22 to 89 years, and body weight ranging from 38 to 123 kg), gender had no effect on the exposure of palbociclib, and age and body weight had no clinically important effect on the exposure of palbociclib.
Paediatric population
Pharmacokinetics of palbociclib has not been evaluated in patients <18 years of age.
Hepatic impairment
Data from a pharmacokinetic trial in subjects with varying degrees of hepatic function indicate that palbociclib unbound exposure (unbound AUCinf) decreased by 17% in subjects with mild hepatic impairment (Child-Pugh class A), and increased by 34% and 77% in subjects with moderate (Child-Pugh class B) and severe (Child-Pugh class C) hepatic impairment, respectively, relative to subjects with normal hepatic function. Peak palbociclib unbound exposure (unbound Cmax) was increased by 7%, 38% and 72% for mild, moderate and severe hepatic impairment, respectively, relative to subjects with normal hepatic function. In addition, based on a population pharmacokinetic analysis that included 183 patients with advanced cancer, where 40 patients had mild hepatic impairment based on National Cancer Institute (NCI) classification (total bilirubin ≤ Upper Limit of Normal (ULN) and Aspartate Aminotransferase (AST) > ULN, or total bilirubin >1.0 to 1.5 × ULN and any AST), mild hepatic impairment had no effect on the pharmacokinetics of palbociclib.
Renal impairment
Data from a pharmacokinetic trial in subjects with varying degrees of renal function indicate that total palbociclib exposure (AUCinf) increased by 39%, 42%, and 31% with mild (60 mL/min≤ CrCl <90 mL/min), moderate (30 mL/min≤ CrCl <60 mL/min), and severe (CrCl <30 mL/min) renal impairment, respectively, relative to subjects with normal (CrCl ≥90 mL/min) renal function. Peak palbociclib exposure (Cmax) was increased by 17%, 12%, and 15% for mild, moderate, and severe renal impairment, respectively, relative to subjects with normal renal function. In addition, based on a population pharmacokinetic analysis that included 183 patients with advanced cancer, where 73 patients had mild renal impairment and 29 patients had moderate renal impairment, mild and moderate renal impairment had no effect on the pharmacokinetics of palbociclib. The pharmacokinetics of palbociclib have not been studied in patients requiring haemodialysis.
Ethnicity
In a pharmacokinetic study in healthy volunteers, palbociclib AUCinf and Cmax values were 30% and 35% higher, respectively, in Japanese subjects compared with non-Asian subjects after a single oral dose. However, this finding was not reproduced consistently in subsequent studies in Japanese or Asian breast cancer patients after multiple dosing. Based on an analysis of the cumulative pharmacokinetic, safety, and efficacy data across Asian and non-Asian populations, no dose adjustment based on Asian race is considered necessary.
Preclinical safety data
The primary target organ findings following single and/or repeat dosing included haematolymphopoietic and male reproductive organ effects in rats and dogs, and effects on bone and actively growing incisors in rats only. These systemic toxicities were generally observed at clinically relevant exposures based on AUC. Partial to full reversal of effects on the hematolymphopoietic, male reproductive systems, and incisor teeth were established, whereas the bone effect was not reversed following a 12-week nondosing period. In addition, cardiovascular effects (QTc prolongation, decreased heart rate, and increased RR interval and systolic blood pressure) were identified in telemetered dogs at ≥4 times human clinical exposure based on Cmax.
Carcinogenicity
Palbociclib was assessed for carcinogenicity in a 6-month transgenic mouse study and in a 2-year rat study. Palbociclib was negative for carcinogenicity in transgenic mice at doses up to 60 mg/kg/day (No Observed Effect Level [NOEL] approximately 11 times human clinical exposure based on AUC). Palbociclib-related neoplastic finding in rats included an increased incidence of microglial cell tumours in the central nervous system of males at 30 mg/kg/day; there were no neoplastic findings in female rats at any dose up to 200 mg/kg/day. The NOEL for palbociclib-related carcinogenicity effects was 10 mg/kg/day (approximately 2 times the human clinical exposure based on AUC) and 200 mg/kg/day (approximately 4 times the human clinical exposure based on AUC) in males and females, respectively. The relevance of the male rat neoplastic finding to humans is unknown.
Genotoxicity
Palbociclib was not mutagenic in a bacterial reverse mutation (Ames) assay and did not induce structural chromosomal aberrations in the in vitro human lymphocyte chromosome aberration assay.
Palbociclib induced micronuclei via an aneugenic mechanism in Chinese Hamster Ovary cells in vitro and in the bone marrow of male rats at doses ≥100 mg/kg/day. The exposure of animals at the no observed effect level for aneugenicity was approximately 7 times human clinical exposure based on AUC.
Impairment of fertility
Palbociclib did not affect mating or fertility in female rats at any dose tested up to 300 mg/kg/day (approximately 3 times human clinical exposure based on AUC), and no adverse effects were observed in female reproductive tissues in repeat-dose toxicity studies up to 300 mg/kg/day in the rat and 3 mg/kg/day in the dog (approximately 5 and 3 times human clinical exposure based on AUC, respectively).
Palbociclib is considered to have the potential to impair reproductive function and fertility in male humans based on non-clinical findings in rats and dogs. Palbociclib-related findings in the testis, epididymis, prostate, and seminal vesicle included decreased organ weight, atrophy or degeneration, hypospermia, intratubular cellular debris, lower sperm motility and density, and decreased secretion. These findings were observed in rats and/or dogs at exposures ≥ 9 times or subtherapeutic compared to human clinical exposure based on AUC, respectively. Partial reversibility of male reproductive organ effects was observed in the rat and dog following a 4- and 12-week nondosing period, respectively. Despite these male reproductive organ findings, there were no effects on mating or fertility in male rats at projected exposure levels 13 times human clinical exposure based on AUC.
Developmental toxicity
Palbociclib is a reversible inhibitor of cyclin-dependent kinases 4 and 6, which are both involved in regulating the cell cycle. It may therefore have risk of foetal harm if used during pregnancy. Palbociclib was foetotoxic in pregnant animals. An increased incidence of a skeletal variation (increased incidence of a rib present at the seventh cervical vertebra) at ≥ 100 mg/kg/day was observed in rats. Reduced foetal body weights were observed at a maternally toxic dose of 300 mg/kg/day in rats (3 times human clinical exposure based on AUC), and an increased incidence of skeletal variations, including small phalanges in the forelimb was observed at a maternally toxic dose of 20 mg/kg/day in rabbits (4 times human clinical exposure based on AUC). Actual foetal exposure and cross-placenta transfer have not been examined.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.