IBU Tablet Ref.[50966] Active ingredients: Ibuprofen
Source: FDA, National Drug Code (US) Revision Year: 2022
Product description
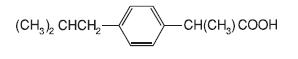
IBU tablets contain the active ingredient ibuprofen, which is (±)2(p-isobutylphenyl) propionic acid. Ibuprofen is a white powde rwith a melting point of 74-77°C and is very slightly soluble in water (<1 mg/mL) and readily soluble in organic solvents such as ethanol and acetone. The structural formula is represented below:
IBU, a nonsteroidal anti-inflammatory drug (NSAID), is available in 400 mg, 600 mg, and 800 mg tablets for oral administration. Inactive ingredients: carnauba wax, colloidal silicon dioxide, croscarmellose sodium, hypromellose, magnesium stearate, microcrystalline cellulose, polydextrose, polyethylene glycol, polysorbate, titanium dioxide.
| How Supplied |
|---|
|
IBU tablets are available in the following strengths, colors and sizes: 600 mg (white, caplet, debossed 6I) Bottles of 100 NDC 66267-964-00 Manufactured by: Dr. Reddy’s Laboratories Louisiana, LLC, Shreveport, LA 71106 USA |
Drugs
| Drug | Countries | |
|---|---|---|
| IBU | Turkey, United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.