INBRIJA Inhalation powder, capsule Ref.[10858] Active ingredients: Levodopa
Source: FDA, National Drug Code (US) Revision Year: 2021
Product description
INBRIJA consists of a dry powder formulation of levodopa for oral inhalation with the INBRIJA inhaler. The inhalation powder is packaged in white hypromellose capsules.
Each capsule contains a spray-dried powder of 42 mg levodopa active ingredient with 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) and sodium chloride.
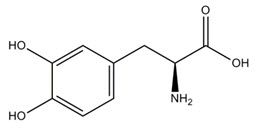
The active component of INBRIJA is levodopa, an aromatic amino acid. Its chemical name is (2S)-2-amino-3-(3,4-dihydroxyphenyl) propanoic acid and its structural formula is:
Levodopa has a molecular weight of 197.19 g/mol and molecular formula C9H11NO4. Levodopa is a white to slightly off-white powder and is readily soluble in formic acid, slightly soluble in water, and practically insoluble in ethanol and diethyl ether; it dissolves in dilute hydrochloric acid.
The INBRIJA inhaler is a plastic device with a blue body, blue cap, and white mouthpiece used for inhaling INBRIJA powder.
The INBRIJA inhaler is breath-actuated by the patient. Under standardized in vitro testing conditions, the INBRIJA inhaler delivered 36.1 mg of levodopa (emitted dose) for the 42 mg capsule from the mouthpiece. No significant difference in emitted dose was observed when varying the flow rate and volume from 20 liters per minute/1L up to 90 liters per minute/2L. Peak inspiratory flow rates (PIFR) achievable through the INBRIJA inhaler were evaluated in 24 adult patients with mild to moderate Parkinson’s disease. The mean PIFR was 64 L/min (range 39–98 L/min) for patients in the ON state and 57 L/min (range 29–98 L/min) in the OFF state.
| Dosage Forms and Strengths |
|---|
|
INBRIJA (levodopa inhalation powder) consists of INBRIJA capsules and the INBRIJA inhaler. INBRIJA capsules contain 42 mg dry powder formulation of levodopa in a white capsule with two black color bands, and “A42” printed on one side. |
| How Supplied |
|---|
|
INBRIJA 42 mg contains foil blister strips of INBRIJA (levodopa inhalation powder) white capsules with two black bands on the body and “A42” in black on the cap, and one INBRIJA inhaler.
INBRIJA inhaler consists of a blue cap, blue handle with “INBRIJA” imprinted on it, and white mouthpiece covering the capsule chamber. Manufactured by: Acorda Therapeutics, Inc., 420 Saw Mill River Road, Ardsley, NY 10502 USA |
Drugs
| Drug | Countries | |
|---|---|---|
| INBRIJA | Austria, Estonia, Croatia, Ireland, Italy, Lithuania, Poland, United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.