INBRIJA Inhalation powder, capsule Ref.[10858] Active ingredients: Levodopa
Source: FDA, National Drug Code (US) Revision Year: 2021
12.1. Mechanism of Action
Levodopa, the metabolic precursor of dopamine, crosses the blood-brain barrier and presumably is converted to dopamine in the brain. This is thought to be the mechanism whereby levodopa relieves symptoms of Parkinson's disease.
12.2. Pharmacodynamics
There are no relevant data on the pharmacodynamic effects of INBRIJA.
12.3. Pharmacokinetics
In the presence of carbidopa, the pharmacokinetics of levodopa are dose-proportional in healthy subjects taking up to 84 mg of INBRIJA. In the presence of carbidopa, the terminal elimination half-life (t1/2) of levodopa following a single administration of INBRIJA 84 mg was 2.3 hours.
Absorption
After a single dose of INBRIJA 84 mg (two 42 mg capsules), the median Tmax for plasma levodopa was approximately 0.5 hours (range 0.17–2.00 hours). In fasted healthy volunteers the bioavailability of levodopa from INBRIJA was approximately 70% relative to immediate-release oral levodopa tablets. The dose-normalized Cmax of levodopa from INBRIJA is approximately 50% of that following immediate-release oral tablets.
Distribution
Apparent volume of distribution (Vz/F) was 168 L for INBRIJA 84 mg.
Metabolism and Elimination
Levodopa is extensively metabolized, and the two major metabolic pathways are decarboxylation by dopa decarboxylase and O-methylation by catechol-O-methyltransferase (COMT).
Specific Populations
Geriatric Population
Clinical studies specifically designed to analyze the effects of age on the pharmacokinetics of levodopa were not conducted with INBRIJA.
Male and Female Patients
After a single dose administration of INBRIJA 84 mg, the body-weight adjusted Cmax and AUC0-24 were similar between women and men. No adjustment in dosage is required based on sex.
Hepatic/Renal Impairment
INBRIJA has not been studied in patients with hepatic or renal impairment.
Smokers
In a pharmacokinetic study following a single administration of INBRIJA 84 mg dose in the presence of carbidopa, levodopa exposure (AUC and Cmax) in smokers (N=25) and non-smokers (N=31) were similar.
13.1. Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
In rats, oral administration of carbidopa/levodopa for two years resulted in no evidence of carcinogenicity.
Mutagenesis
Studies to assess the potential mutagenic or clastogenic effects of levodopa have not been conducted.
Impairment of Fertility
In reproduction studies in rats, oral administration of carbidopa/levodopa resulted in no effects on fertility.
14. Clinical Studies
The efficacy and safety of INBRIJA for the treatment of OFF episodes in patients with Parkinson's disease treated with oral carbidopa/levodopa was evaluated in a 12-week, randomized, placebo-controlled, double-blind study (Study 1; NCT02240030).
Study 1
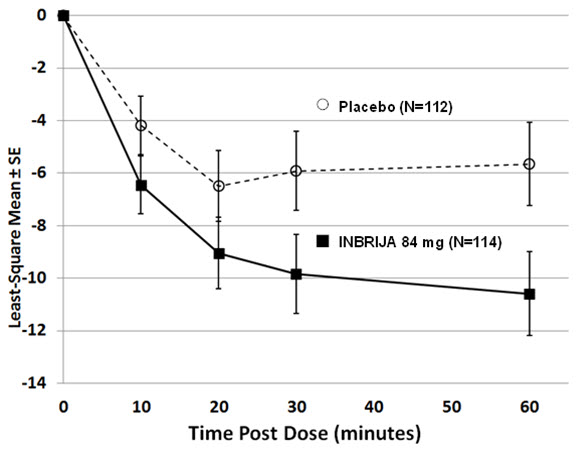
In Study 1, a total of 114 patients were treated with INBRIJA 84 mg (two 42 mg capsules), and 112 patients received placebo. Study medication could be administered up to five times a day. At baseline, patients had at least 2 hours of OFF time per day, and carbidopa/levodopa medication did not exceed 1600 mg levodopa per day. The mean UPDRS Part III scores at screening in the ON state were 14.9 for patients randomized to INBRIJA 84 mg and 16.1 for patients randomized to placebo. The UPDRS part III is designed to assess the severity of the cardinal motor findings (e.g., tremor, rigidity, bradykinesia, postural instability) in patients with Parkinson's disease.
The primary endpoint was the change in Unified Parkinson's Disease Rating Scale (UPDRS) Part III motor score from pre-dose OFF state to 30 minutes post-dose, measured at Week 12. The average use of INBRIJA 84 mg or placebo was approximately 2 doses per day. At Week 12, the reduction in UPDRS Part III motor score for INBRIJA 84 mg, compared to placebo at 30 minutes post-dose, were -9.8 and -5.9, respectively (See Table 2 and Figure 1). The proportion of patients who returned to an ON state and sustained that ON through 60 minutes post-dose was 58% for INBRIJA 84 mg and 36% for placebo (p=0.003).
Table 2. Mean Change in UPDRS Part III Motor Score at 30 minutes post-dose (INBRIJA 84 mg) for the Intent-to-Treat Population at Week 12*:
| Treatment | Pre-dose (OFF) UPDRS Part III Motor Score (mean) | Post-dose UPDRS Part III Motor Score (mean) | Mean Change 30 minutes post-dose†,‡ | Difference from Placebo (95% confidence interval) | p-value |
|---|---|---|---|---|---|
| Placebo | 32.1 | 25.3 | -5.9 | — | — |
| INBRIJA 84 mg | 29.0 | 19.3 | -9.8 | -3.92 (-6.84, -1.00) | 0.009 |
Figure 1. Least-squares Mean Change in UPDRS Part III Motor Score After Administration of INBRIJA 84 mg vs. Placebo (at Week 12):
Study 2
The effect of INBRIJA on pulmonary function was evaluated in patients with Parkinson's disease treated with oral carbidopa/levodopa in a 12 month, randomized, controlled, open-labeled study (Study 2: NCT02352363). A total of 271 patients were treated with INBRIJA 84 mg (two 42 mg capsules), and 127 patients with Parkinson's disease in a control group were observed on their regular oral medication regimen for the treatment of Parkinson's disease. Patients with chronic obstructive pulmonary disease (COPD), asthma, or other chronic respiratory disease within the last 5 years were excluded [see Warnings and Precautions (5.6)]. Pulmonary function was assessed by spirometry every 3 months in both groups. After 12 months, the average reduction in the forced expiratory volume in 1 second (FEV1) from baseline was the same in both groups (-0.1 L).
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.