ISOPTO ATROPINE Ophthalmic solution Ref.[10851] Active ingredients: Atropine
Source: FDA, National Drug Code (US) Revision Year: 2020
Product description
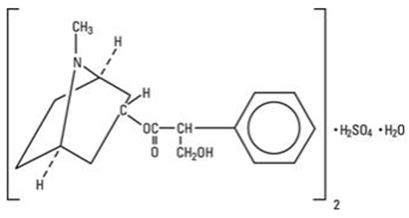
ISOPTO Atropine 1% is a sterile topical ophthalmic solution. Each mL of ISOPTO Atropine 1% contains 10 mg of atropine sulfate monohydrate equivalent to 9.7 mg/mL of atropine sulfate or 8.3 mg of atropine. Atropine sulfate monohydrate is designated chemically as benzeneacetic acid, α-(hydroxymethyl)-,8-methyl-8-aza-bicyclo-[3.2.1]oct-3-yl ester, endo(+), sulfate(2:1) (salt), monohydrate.
Its molecular formula is (C17H23NO3)2•H2SO4•H2O and it is represented by the chemical structure:
Atropine sulfate monohydrate is colorless crystals or white crystalline powder and has a molecular weight of 694.83.
ISOPTO Atropine 1% has a pH of 3.5 to 6.0.
Active ingredient: atropine sulfate monohydrate 1.0%
Preservative: benzalkonium chloride 0.01%
Inactive ingredients: hypromellose, boric acid, sodium hydroxide and/or hydrochloric acid (to adjust pH), purified water.
| Dosage Forms and Strengths |
|---|
|
Ophthalmic solution: 1% atropine sulfate (10mg/mL). |
| How Supplied |
|---|
|
ISOPTO Atropine 1% is supplied sterile in low-density polyethylene plastic DROP-TAINER dispensers with low-density polyethylene tips and red polypropylene caps as follows:
ALCON, A Novartis company, Fort Worth, Texas 76134 USA |
Drugs
| Drug | Countries | |
|---|---|---|
| ISOPTO ATROPINE | Canada, Estonia, United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.