JESDUVROQ Film coated tablet Ref.[50977] Active ingredients: Daprodustat
Source: FDA, National Drug Code (US) Revision Year: 2023
Product description
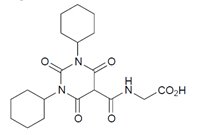
JESDUVROQ contains daprodustat, an inhibitor of hypoxia inducible factor (HIF), prolyl 4-hydroxylases (PH)1, PH2 and PH3. The chemical name of daprodustat is N‑[(1,3‑dicyclohexylhexahydro-2,4,6-trioxopyrimidin-5-yl) carbonyl]glycine. The molecular formula of daprodustat is C19H27N3O6, and its molecular mass is 393.43. The structural formula is shown below.
Daprodustat is a white to off-white powder that is poorly soluble in water.
Each JESDUVROQ oral tablet contains 1 mg, 2 mg, 4 mg, 6 mg, or 8 mg of daprodustat. Inactive ingredients include colloidal silicon dioxide, croscarmellose sodium, hypromellose, magnesium stearate, mannitol and microcrystalline cellulose. The tablet film-coating inactive ingredients include hypromellose, iron oxide black (1 mg, 2 mg, and 6 mg tablets), iron oxide red and iron oxide yellow (1 mg, 2 mg, 6 mg, and 8 mg tablets), polyethylene glycol, and titanium dioxide.
| Dosage Forms and Strengths |
|---|
|
Tablets:
|
| How Supplied | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
JESDUVROQ tablets contain 1 mg, 2 mg, 4 mg, 6 mg or 8 mg of daprodustat.
Manufactured by, GlaxoSmithKline, Durham, NC 27701 |
Drugs
| Drug | Countries | |
|---|---|---|
| JESDUVROQ | United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.