JETREA Concentrate for solution for injection Ref.[9656] Active ingredients: Ocriplasmin
Source: European Medicines Agency (EU) Revision Year: 2019 Publisher: Oxurion NV, Gaston Geenslaan 1, B-3001, Leuven, Belgium
Pharmacodynamic properties
Pharmacotherapeutic group: Ophthalmologicals, Other ophthalmologicals
ATC code: S01XA22
Mechanism of action
Ocriplasmin has a proteolytic activity against protein components of the vitreous body and the vitreoretinal interface (VRI) (e.g. laminin, fibronectin and collagen) and aims to dissolve the protein matrix responsible for the abnormal vitreomacular adhesion (VMA). The tight binding of the protein components within the macular area of the VRI contribute to vitreomacular traction (VMT), leading to visual impairment and/or macular holes.
Clinical efficacy and safety
The clinical efficacy and safety of JETREA for the treatment of vitreomacular traction (VMT) was assessed in 3 double-masked studies.
Studies TG-MV-006 and TG MV-007
The efficacy of JETREA was demonstrated in 2 pivotal multicentre, randomised, double-masked, placebo-controlled, 6-month studies in patients with VMT. A total of 652 patients (JETREA 464, placebo 188) were randomised in these 2 studies.
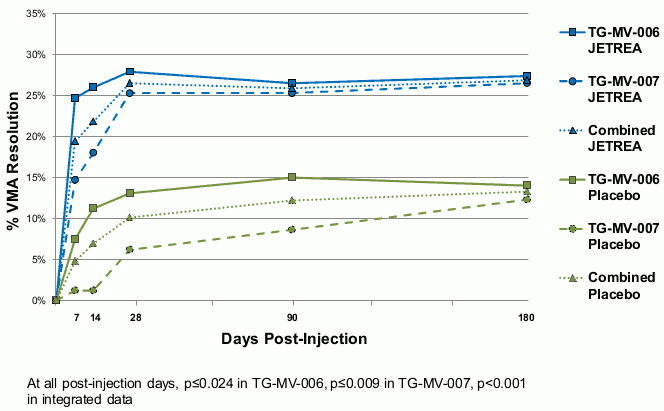
In both pivotal studies, the proportion of patients who achieved VMA resolution at Day 28 (primary endpoint) was significantly (p≤0.003) higher in the JETREA group compared with the placebo group. The difference continued to be statistically significant through Month 6 in each study (p≤0.024). In the integrated data, 26.5% in the JETREA group compared with 10.1% in the placebo group achieved VMA resolution at Day 28 (p<0.001). The difference was maintained from Day 7 through Month 6 (Figure 1).
Figure 1. Proportion of patients with VMA resolution up to Day 180 (Month 6) (TG-MV-006, TG-MV-007 and integrated data):
Patients with no ERM at baseline were more likely to achieve VMA resolution at Day 28 compared with those who had ERM at baseline. In the integrated data, the VMA resolution rate at Day 28 was higher in patients treated with JETREA compared to placebo in both the subgroup without ERM (37.4% vs. 14.3%, p<0.001) and with ERM (8.7% vs. 1.5%, p=0.046).
Patients with a smaller VMA diameter at baseline (≤1500 microns) were more likely to achieve VMA resolution at Day 28 compared with those who had a diameter >1500 microns. In the integrated data, the VMA resolution rate at Day 28 was higher in patients treated with JETREA compared to placebo in both the subgroup with VMA ≤1500 microns at baseline (34.7% vs. 14.6%, p<0.001) and with VMA >1500 microns at baseline (5.9% vs. 0%, p=0.113).
In the integrated data, full-thickness macular hole (FTMH) was present at baseline in 106/464 (22.8%) patients and 47/188 (25%) patients in the JETREA and placebo groups, respectively. Of these, the proportion of patients who achieved FTMH closure without vitrectomy at Day 28 was higher in the JETREA group than the placebo group (40.6% vs. 10.6%, respectively; p<0.001). A difference was maintained through the end of the studies (Month 6).
A significantly higher percentage of JETREA treated patients experienced total PVD at Day 28 compared to placebo treated patients (integrated data: 13.4% vs. 3.7%, respectively; p<0.001).
During the studies, vitrectomy could be performed at the discretion of the Investigator. JETREA treated patients were less likely to have had a vitrectomy by the end of the study (Month 6) compared with placebo treated patients (integrated data: 17.7% vs. 26.6%, respectively; p=0.016).
A higher proportion of JETREA treated patients gained ≥2 or ≥3 lines in BCVA (irrespective of vitrectomy) at Month 6 (28.0% and 12.3%, respectively) compared with patients treated with placebo (17.1% and 6.4%) (p=0.003 and p=0.024, respectively). Also the proportion of patients gaining ≥2 or ≥3 lines in BCVA without vitrectomy favoured JETREA at Month 6 (23.7% vs. 11.2%, p<0.001 for a gain ≥2 lines and 9.7% vs. 3.7%, p=0.008 for a gain ≥3 lines).
In the integrated analysis of the National Eye Institute Visual Function Questionnaire-25 (VFQ-25), a numerical difference in favour of JETREA over placebo was shown in each sub-scale score, as well as the composite score. The difference for improvement in the general vision sub-scale score was statistically significant (6.1 JETREA vs. 2.1 placebo, p=0.024).
Study TG-MV-014
The efficacy of JETREA has been further confirmed in a randomised, double-masked, shamcontrolled, 24-month study in patients with VMT finalised since the initial marketing authorisation approval. A total of 220 patients (JETREA 146, sham 74) were randomised in this study.
The proportion of patients who achieved VMA resolution at Day 28 (primary endpoint) was 41.7% in the JETREA group compared with 6.2% in the sham group (p<0.001). This effect was maintained over time and VMA resolution was consistently greater in the JETREA group at each post-injection study visit compared with the sham group.
In this study, FTMH was present at baseline in 50/145 (34.5%) and 26/73 (35.6%) patients in the JETREA and sham groups, respectively. Of these, 30% of JETREA treated patients and 15.4% of patients in the sham group experienced non-surgical FTMH closure at Month 24. All had done so by Month 3.
The proportion of patients who underwent vitrectomy was smaller in the JETREA group than in the sham group at all visits. At Month 24, the proportions were 48/145 (33.3%) and 32/73 (43%), respectively. The most common reason for performing vitrectomy was FTMH (in 24.8% JETREA treated patients and 23.3 % sham patients). The proportion of patients who underwent vitrectomy for an event of VMA/VMT was 8.3% in the JETREA group compared to 19.2% in the sham group.
The proportion of patients who gained ≥2 or ≥3 lines in BCVA at Month 6, irrespective of vitrectomy, was slightly higher in the JETREA group (36.2%, 18.6%) than in the sham group (28.6%, 13.1%). At Month 24, the proportion of patients with ≥2 lines improvement from baseline in BCVA was greater in the JETREA group than in the sham group (50.5% vs. 39.1%). The proportion of patients with ≥3 lines improvement from baseline was only greater in the JETREA group (23.4% vs. 12.8%, respectively) in the subgroup who had no FTMH at baseline. The ≥2 or ≥3 lines gain in BCVA without vitrectomy favoured JETREA over sham both at Month 6 (26.8%, 14.0%, vs. 15.62%, 6.2%, respectively) and Month 24 (31.9%, 16.8%, vs. 11,7%, 4.1%, respectively).
A greater proportion of patients in the JETREA group had a ≥5 points improvement in VFQ-25 composite and sub-scale scores, irrespective of vitrectomy, at all visits. At Month 24, 51.4% of JETREA patients had a ≥5 points improvement in VFQ-25 composite score compared to 30.1% in the sham group.
Paediatric population
The European Medicines Agency has waived the obligation to submit the results of studies with JETREA in all subsets of the paediatric population in the treatment of vitreomacular traction (VMT), including when associated with macular hole of diameter less than or equal to 400 microns (see section 4.2 for information on paediatric use).
The safety and efficacy of ocriplasmin in paediatric subjects scheduled for vitrectomy was investigated in study TG-MV-009. A single intravitreal injection of 0.175 mg (above the recommended dose), or placebo, was injected in the mid-vitreous of 24 eyes of children aged 0 to 16 years, 30 to 60 minutes prior to the planned start of vitrectomy. The main reasons for vitrectomy were retinal detachment and retinopathy of prematurity. Treatment with ocriplasmin did not demonstrate an effect on posterior vitreous detachment rate, vitreous liquefaction grade, immediate postoperative retinal reattachment rate, development of proliferative vitreoretinopathy, or stage of retinopathy of prematurity. The safety findings observed in study TG-MV-009 were consistent with the known safety profile for JETREA. Based on the results of this study, the use of JETREA as an adjunct to vitrectomy in children, to facilitate vitreous separation and removal, is not recommended.
Ethnicity
Experience is limited in groups other than Caucasians.
Pharmacokinetic properties
Ocriplasmin levels in the vitreous decrease rapidly after intravitreal administration. In a clinical study in patients scheduled for vitrectomy receiving 0.125 mg JETREA (corresponding to a theoretical start concentration of 29 µg/mL vitreous), mean ocriplasmin activity was 9% of theoretical start concentration 2-4 hours after injection and below the lower level of quantification at 7 days.
Because of the small dose administered (0.125 mg), detectable levels of ocriplasmin in systemic circulation are not expected after intravitreal injection.
When administered intravenously, ocriplasmin enters the endogenous protein catabolism pathway through which it is rapidly inactivated via its interactions with protease inhibitor α2-antiplasmin or α2-macroglobulin. The inactive ocriplasmin/α2-antiplasmin complex is cleared from the circulation with a half-life (t1/2) of several hours.
Renal impairment
No studies have been conducted to examine the pharmacokinetics of ocriplasmin in patients with renal impairment since the systemic exposure is expected to be very low after intravitreal administration.
Hepatic impairment
No studies have been conducted to examine the pharmacokinetics of ocriplasmin in patients with hepatic impairment since the systemic exposure is expected to be very low after intravitreal administration.
Preclinical safety data
The intravitreal toxicity of ocriplasmin has been evaluated in rabbits, monkeys and minipigs. Ocriplasmin induced an inflammatory response and transient ERG changes in rabbits and monkeys, while no inflammation or ERG changes were observed in minipigs. In rabbits and monkeys, the incidence of vitreous cell infiltrates tended to resolve over time. In monkeys, after administration of 125 µg/eye (68 µg/mL vitreous) the ERG was fully recovered by Day 55. Lens subluxation was observed in the 3 species at ocriplasmin concentrations at or above 41 µg/mL vitreous, a concentration above the intended clinical concentration of 29 µg/mL. This effect appeared to be dose-related and was observed in all animals administered intravitreal ocriplasmin more than once. Pathological changes related to intraocular haemorrhage were observed in rabbits and monkeys. It remains unclear if this haemorrhage is related to the injection procedure itself or administration of ocriplasmin. No systemic toxicity was observed after intravitreal administration of ocriplasmin.
The systemic toxicity of ocriplasmin has been evaluated in both rat and dog. Intravenous administration of 10 mg/kg was generally well tolerated in both rat and dog whether administered as single dose or as repeated dose.
No carcinogenicity, mutagenicity or reproductive and developmental toxicity data are available.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.