JETREA Concentrate for solution for injection Ref.[9656] Active ingredients: Ocriplasmin
Source: European Medicines Agency (EU) Revision Year: 2019 Publisher: Oxurion NV, Gaston Geenslaan 1, B-3001, Leuven, Belgium
Therapeutic indications
JETREA is indicated in adults for the treatment of vitreomacular traction (VMT), including when associated with macular hole of diameter less than or equal to 400 microns (see section 5.1).
Posology and method of administration
JETREA must be prepared and administered by a qualified ophthalmologist experienced in intravitreal injections. The diagnosis of vitreomacular traction (VMT) should comprise of a complete clinical picture including patient history, clinical examination and investigation using currently accepted diagnostic tools, such as optical coherence tomography (OCT).
Posology
The recommended dose is 0.125 mg (0.1 mL of the diluted solution) administered by intravitreal injection to the affected eye once as a single dose. Each vial should only be used once and for the treatment of a single eye. Treatment with JETREA in the other eye is not recommended concurrently or within 7 days of the initial injection in order to monitor the post-injection course including the potential for decreased vision in the injected eye. Repeated administration in the same eye is not recommended (see section 4.4).
See section 4.4 for instructions on post-injection monitoring.
Special populations
Renal impairment
No formal studies have been conducted with JETREA in patients with renal impairment. No dose adjustment or special considerations are anticipated for patients with renal impairment (see section 5.2).
Hepatic impairment
No formal studies have been conducted with JETREA in patients with hepatic impairment. No dose adjustment or special considerations are anticipated for patients with hepatic impairment (see section 5.2).
Elderly
The elderly population has been studied in clinical studies. No dose adjustment is required.
Paediatric population
There is no relevant use of JETREA in children aged under 18 years for the indication of vitreomacular traction (VMT), including when associated with macular hole of diameter less than or equal to 400 microns. Currently available data on paediatric use are described in section 5.1.
Method of administration
Single use vial for intravitreal use only.
Preoperative antibiotic drops may be administered at the discretion of the treating ophthalmologist.
Precautions to be taken before handling or administering the medicinal product
The intravitreal injection procedure should be carried out under controlled aseptic conditions, which include the use of surgical hand disinfection, sterile gloves, a sterile drape, a sterile eyelid speculum (or equivalent) and the availability of sterile paracentesis (if required). The periocular skin, eyelid and ocular surface should be disinfected and adequate anaesthesia and a broad spectrum topical microbiocide should be administered prior to the injection according to standard medical practice.
For instructions on dilution of the medicinal product before administration, see section 6.6.
The injection needle should be inserted 3.5-4.0 mm posterior to the limbus aiming towards the centre of the vitreous cavity avoiding the horizontal meridian. The injection volume of 0.1 mL is then delivered into the mid-vitreous.
Overdose
The clinical data on the effects of JETREA overdose are limited. One case of accidental overdose of 0.250 mg ocriplasmin (twice the recommended dose) has been reported. The patient had a decrease in BCVA of 21 ETDRS letters from baseline that returned to within 9 letters of baseline at the end of the study. The patient also developed mild conjunctival hyperaemia, eye inflammation and miosis which resolved with corticosteroid eye drops.
If an overdose occurs, close monitoring is recommended. If an adverse reaction occurs, it should be treated according to standard medical practice.
Shelf life
3 years when stored in a freezer (-20°C ± 5°C).
After thawing: The medicinal product should be diluted and used immediately. However, chemical and physical in-use stability of unopened product in the original carton protected from light has been demonstrated for up to 8 hours when stored below 25°C. Do not refreeze a vial once it has been thawed.
After opening/dilution: From a microbiological point of view, the medicinal product must be used immediately after opening/dilution. The vial and any unused portion of the diluted solution must be discarded after single use.
Special precautions for storage
Store in a freezer (-20°C ± 5°C).
For storage conditions after thawing and opening/dilution of the medicinal product, see section 6.3.
Nature and contents of container
0.2 mL solution in a vial (type I glass) closed with a chlorobutyl rubber stopper and an orange polypropylene flip-off cap. Pack containing 1 vial.
Special precautions for disposal and other handling
Vials are for single use only.
To prepare JETREA for intravitreal injection, adhere to the following instructions:
1. Remove the vial from the freezer and allow to thaw at room temperature (takes about 2 minutes).
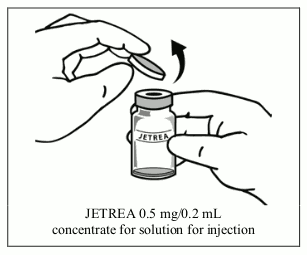
2. Once completely thawed, remove the protective orange polypropylene flip-off cap from the vial.
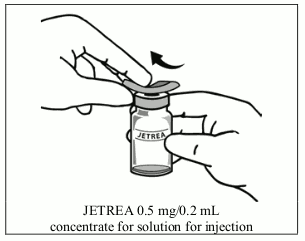
3. Disinfect the top of the vial with an alcohol wipe.
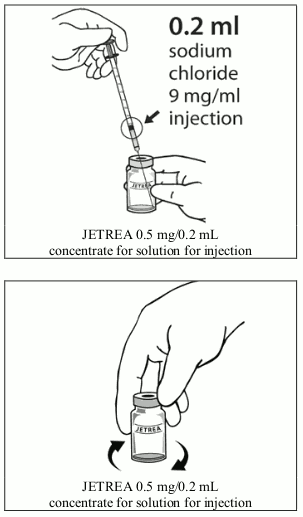
4. Using aseptic technique, dilute by adding 0.2 mL of sodium chloride 9 mg/mL (0.9%) solution for injection (sterile, preservative-free, non-buffered) into the JETREA vial and gently swirl the vial until the solutions are mixed. The diluent should be withdrawn from an unopened container which should be used only once. The remaining sodium chloride 9 mg/mL (0.9%) solution for injection should be discarded. The diluted solution should be used immediately.
5. Visually inspect the vial for particulate matter. Only a clear, colourless solution without visible particles should be used.
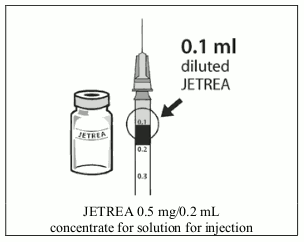
6. Using aseptic technique, withdraw all of the diluted solution using an appropriate sterile needle (slightly incline the vial to ease withdrawal) and discard the needle after withdrawal of the vial contents. Do not use this needle for the intravitreal injection.
7. Replace the needle with an appropriate sterile needle, carefully expel the excess volume from the syringe by slowly depressing the plunger so that the plunger tip aligns with the 0.1 mL line on the syringe (corresponding to 0.125 mg ocriplasmin).
8. Inject 0.1 mL of the diluted solution immediately into the mid-vitreous.
9. Discard the vial and any unused portion of the diluted solution after single use.
Any unused medicinal product or waste material should be disposed of in accordance with local requirements.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.