KOMBIGLYZE XR Film-coated tablet Ref.[50760] Active ingredients: Metformin Saxagliptin
Source: FDA, National Drug Code (US) Revision Year: 2019
Product description
KOMBIGLYZE XR (saxagliptin and metformin HCl extended-release) tablets contain two oral antihyperglycemic medications used in the management of type 2 diabetes: saxagliptin and metformin hydrochloride.
Saxagliptin
Saxagliptin is an orally active inhibitor of the dipeptidyl-peptidase-4 (DPP4) enzyme.
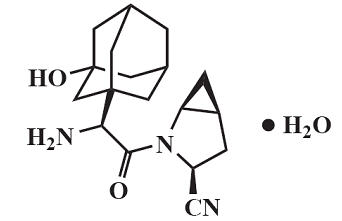
Saxagliptin monohydrate is described chemically as (1S,3S,5S)2[(2S)-2-Amino-2-(3-hydroxytricyclo[3.3.1.1 3,7]dec-1-yl)acetyl]-2-azabicyclo[3.1.0]hexane-3-carbonitrile, monohydrate or (1S,3S,5S)2[(2S)-2-Amino-2-(3-hydroxyadamantan-1-yl)acetyl]-2-azabicyclo[3.1.0]hexane-3-carbonitrile hydrate. The empirical formula is C18H25N3O2•H2O and the molecular weight is 333.43.
The structural formula is:
Saxagliptin monohydrate is a white to light yellow or light brown, non-hygroscopic, crystalline powder. It is sparingly soluble in water at 24°C ± 3°C, slightly soluble in ethyl acetate, and soluble in methanol, ethanol, isopropyl alcohol, acetonitrile, acetone, and polyethylene glycol 400 (PEG 400).
Metformin hydrochloride
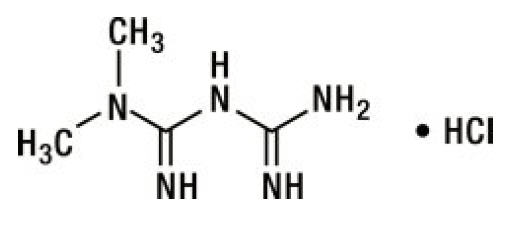
Metformin hydrochloride (N,N-dimethylimidodicarbonimidic diamide hydrochloride) is a white to off-white crystalline compound with a molecular formula of C4H11N5 • HCl and a molecular weight of 165.63. Metformin hydrochloride is freely soluble in water, slightly soluble in alcohol, and is practically insoluble in acetone, ether, and chloroform. The pKa of metformin is 12.4. The pH of a 1% aqueous solution of metformin hydrochloride is 6.68.
The structural formula is:
KOMBIGLYZE XR
KOMBIGLYZE XR is available for oral administration as tablets containing either 5.58 mg saxagliptin hydrochloride (anhydrous) equivalent to 5 mg saxagliptin and 500 mg metformin hydrochloride (KOMBIGLYZE XR 5 mg/500 mg), or 5.58 mg saxagliptin hydrochloride (anhydrous) equivalent to 5 mg saxagliptin and 1000 mg metformin hydrochloride (KOMBIGLYZE XR 5 mg/1000 mg), or 2.79 mg saxagliptin hydrochloride (anhydrous) equivalent to 2.5 mg saxagliptin and 1000 mg metformin hydrochloride (KOMBIGLYZE XR 2.5 mg/1000 mg).
Each film-coated tablet of KOMBIGLYZE XR contains the following inactive ingredients: carboxymethylcellulose sodium, hypromellose 2208, and magnesium stearate. The 5 mg/500 mg strength tablet of KOMBIGLYZE XR also contains microcrystalline cellulose and hypromellose 2910. In addition, the film coatings contain the following inactive ingredients: polyvinyl alcohol, polyethylene glycol 3350, titanium dioxide, talc, and iron oxides.
The biologically inert components of the tablet may occasionally remain intact during gastrointestinal transit and will be eliminated in the feces as a soft, hydrated mass.
| Dosage Forms and Strengths |
|---|
|
| How Supplied | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
KOMBIGLYZE XR (saxagliptin and metformin HCl extended-release) tablets have markings on both sides and are available in the strengths and packages listed in Table 15. Table 15. KOMBIGLYZE XR Tablet Presentations:
Distributed by: AstraZeneca Pharmaceuticals LP, Wilmington, DE 19850 |
Drugs
| Drug | Countries | |
|---|---|---|
| KOMBIGLYZE XR | Australia, Brazil, Ecuador, Hong Kong, Mexico, New Zealand, Singapore, Tunisia, United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.