KOMBIGLYZE XR Film-coated tablet Ref.[50760] Active ingredients: Metformin Saxagliptin
Source: FDA, National Drug Code (US) Revision Year: 2019
12.1. Mechanism of Action
Saxagliptin
Increased concentrations of the incretin hormones such as glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) are released into the bloodstream from the small intestine in response to meals. These hormones cause insulin release from the pancreatic beta cells in a glucose-dependent manner but are inactivated by the DPP4 enzyme within minutes. GLP-1 also lowers glucagon secretion from pancreatic alpha cells, reducing hepatic glucose production. In patients with type 2 diabetes, concentrations of GLP-1 are reduced but the insulin response to GLP-1 is preserved. Saxagliptin is a competitive DPP4 inhibitor that slows the inactivation of the incretin hormones, thereby increasing their bloodstream concentrations and reducing fasting and postprandial glucose concentrations in a glucose-dependent manner in patients with type 2 diabetes mellitus.
Metformin hydrochloride
Metformin improves glucose tolerance in patients with type 2 diabetes, lowering both basal and postprandial plasma glucose. Metformin decreases hepatic glucose production, decreases intestinal absorption of glucose, and improves insulin sensitivity by increasing peripheral glucose uptake and utilization. Unlike sulfonylureas, metformin does not produce hypoglycemia in patients with type 2 diabetes or in healthy subjects except in unusual circumstances [see Warnings and Precautions (5.6)] and does not cause hyperinsulinemia. With metformin therapy, insulin secretion remains unchanged while fasting insulin levels and day-long plasma insulin response may actually decrease.
KOMBIGLYZE XR
KOMBIGLYZE XR combines two antihyperglycemic medications with complementary mechanisms of action to improve glycemic control in adults with type 2 diabetes: saxagliptin, a dipeptidyl-peptidase-4 (DPP4) inhibitor, and metformin hydrochloride, a biguanide.
12.2. Pharmacodynamics
Saxagliptin
In patients with type 2 diabetes mellitus, administration of saxagliptin inhibits DPP4 enzyme activity for a 24-hour period. After an oral glucose load or a meal, this DPP4 inhibition resulted in a 2- to 3-fold increase in circulating levels of active GLP-1 and GIP, decreased glucagon concentrations, and increased glucose-dependent insulin secretion from pancreatic beta cells. The rise in insulin and decrease in glucagon were associated with lower fasting glucose concentrations and reduced glucose excursion following an oral glucose load or a meal.
Cardiac Electrophysiology
Saxagliptin
In a randomized, double-blind, placebo-controlled, 4-way crossover, active comparator study using moxifloxacin in 40 healthy subjects, saxagliptin was not associated with clinically meaningful prolongation of the QTc interval or heart rate at daily doses up to 40 mg (8-times the MRHD).
12.3. Pharmacokinetics
KOMBIGLYZE XR
Bioequivalence and food effect of KOMBIGLYZE XR was characterized under low calorie diet. The low calorie diet consisted of 324 kcal with meal composition that contained 11.1% protein, 10.5% fat, and 78.4% carbohydrate. The results of bioequivalence studies in healthy subjects demonstrated that KOMBIGLYZE XR combination tablets are bioequivalent to coadministration of corresponding doses of saxagliptin (ONGLYZA ) and metformin hydrochloride extended-release (GLUCOPHAGE XR) as individual tablets under fed conditions.
Saxagliptin
The pharmacokinetics of saxagliptin and its active metabolite, 5-hydroxy saxagliptin were similar in healthy subjects and in patients with type 2 diabetes mellitus. The Cmax and AUC values of saxagliptin and its active metabolite increased proportionally in the 2.5 to 400 mg dose range. Following a 5 mg single oral dose of saxagliptin to healthy subjects, the mean plasma AUC values for saxagliptin and its active metabolite were 78 ng•h/mL and 214 ng•h/mL, respectively. The corresponding plasma Cmax values were 24 ng/mL and 47 ng/mL, respectively. The average variability (CV) for AUC and Cmax for both saxagliptin and its active metabolite was less than 25.
No appreciable accumulation of either saxagliptin or its active metabolite was observed with repeated once-daily dosing at any dose level. No dose- and time-dependence were observed in the clearance of saxagliptin and its active metabolite over 14 days of once-daily dosing with saxagliptin at doses ranging from 2.5 to 400 mg.
Metformin hydrochloride
Metformin extended-release Cmax is achieved with a median value of 7 hours and a range of 4 to 8 hours. At steady state, the AUC and Cmax are less than dose proportional for metformin extended-release within the range of 500 to 2000 mg. After repeated administration of metformin extended-release, metformin did not accumulate in plasma. Metformin is excreted unchanged in the urine and does not undergo hepatic metabolism. Peak plasma levels of metformin extended-release tablets are approximately 20% lower compared to the same dose of metformin immediate-release tablets, however, the extent of absorption (as measured by AUC) is similar between extended-release tablets and immediate-release tablets.
Absorption
Saxagliptin
The median time to maximum concentration (Tmax) following the 5 mg once daily dose was 2 hours for saxagliptin and 4 hours for its active metabolite. Administration with a high-fat meal resulted in an increase in Tmax of saxagliptin by approximately 20 minutes as compared to fasted conditions. There was a 27% increase in the AUC of saxagliptin when given with a meal as compared to fasted conditions. Food has no significant effect on the pharmacokinetics of saxagliptin when administered as KOMBIGLYZE XR combination tablets.
Metformin hydrochloride
Following a single oral dose of metformin extended-release, Cmax is achieved with a median value of 7 hours and a range of 4 to 8 hours. Although the extent of metformin absorption (as measured by AUC) from the metformin extended-release tablet increased by approximately 50% when given with food, there was no effect of food on Cmax and Tmax of metformin. Both high and low fat meals had the same effect on the pharmacokinetics of metformin extended-release. Food has no significant effect on the pharmacokinetics of metformin when administered as KOMBIGLYZE XR combination tablets.
Distribution
Saxagliptin
The in vitro protein binding of saxagliptin and its active metabolite in human serum is negligible. Therefore, changes in blood protein levels in various disease states (e.g., renal or hepatic impairment) are not expected to alter the disposition of saxagliptin.
Metformin hydrochloride
Distribution studies with extended-release metformin have not been conducted; however, the apparent volume of distribution (V/F) of metformin following single oral doses of immediate-release metformin 850 mg averaged 654 ± 358 L. Metformin is negligibly bound to plasma proteins, in contrast to sulfonylureas, which are more than 90% protein bound. Metformin partitions into erythrocytes, most likely as a function of time. Metformin is negligibly bound to plasma proteins and is, therefore, less likely to interact with highly protein-bound drugs such as salicylates, sulfonamides, chloramphenicol, and probenecid, as compared to the sulfonylureas, which are extensively bound to serum proteins.
Metabolism
Saxagliptin
The metabolism of saxagliptin is primarily mediated by cytochrome P450 3A4/5 (CYP3A4/5). The major metabolite of saxagliptin is also a DPP4 inhibitor, which is one-half as potent as saxagliptin. Therefore, strong CYP3A4/5 inhibitors and inducers will alter the pharmacokinetics of saxagliptin and its active metabolite [see Drug Interactions (7.1)].
Metformin hydrochloride
Intravenous single-dose studies in healthy subjects demonstrate that metformin is excreted unchanged in the urine and does not undergo hepatic metabolism (no metabolites have been identified in humans) or biliary excretion.
Metabolism studies with extended-release metformin tablets have not been conducted.
Excretion
Saxagliptin
Saxagliptin is eliminated by both renal and hepatic pathways. Following a single 50 mg dose of 14C-saxagliptin, 24%, 36%, and 75% of the dose was excreted in the urine as saxagliptin, its active metabolite, and total radioactivity, respectively. The average renal clearance of saxagliptin (~230 mL/min) was greater than the average estimated glomerular filtration rate (~120 mL/min), suggesting some active renal excretion. A total of 22% of the administered radioactivity was recovered in feces representing the fraction of the saxagliptin dose excreted in bile and/or unabsorbed drug from the gastrointestinal tract. Following a single oral dose of saxagliptin 5 mg to healthy subjects, the mean plasma terminal half-life (t1/2) for saxagliptin and its active metabolite was 2.5 and 3.1 hours, respectively.
Metformin hydrochloride
Renal clearance is approximately 3.5 times greater than creatinine clearance, which indicates that tubular secretion is the major route of metformin elimination. Following oral administration, approximately 90% of the absorbed drug is eliminated via the renal route within the first 24 hours, with a plasma elimination half-life of approximately 6.2 hours. In blood, the elimination half-life is approximately 17.6 hours, suggesting that the erythrocyte mass may be a compartment of distribution.
Specific Populations
Renal Impairment
Saxagliptin:
A single-dose, open-label study was conducted to evaluate the pharmacokinetics of saxagliptin (10 mg dose) in subjects with varying degrees of chronic renal impairment compared to subjects with normal renal function. The 10 mg dosage is not an approved dosage. The degree of renal impairment did not affect Cmax of saxagliptin or its metabolite. In subjects with moderate renal impairment with eGFR 30 to less than 45 mL/min/1.73 m 2, severe renal impairment (eGFR 15 to less than 30 mL/min/1.73 m 2) and ESRD patient on hemodialysis, the AUC values of saxagliptin or its active metabolite were >2 fold higher than AUC values in subjects with normal renal function.
Metformin hydrochloride:
In patients with decreased renal function, the plasma and blood half-life of metformin is prolonged and the renal clearance is decreased [see Contraindications (4) and Warnings and Precautions (5.1)].
Hepatic Impairment
No pharmacokinetic studies of metformin have been conducted in patients with hepatic impairment.
Body Mass Index
Saxagliptin:
No dosage adjustment is recommended based on body mass index (BMI) which was not identified as a significant covariate on the apparent clearance of saxagliptin or its active metabolite in the population pharmacokinetic analysis.
Gender
Saxagliptin:
No dosage adjustment is recommended based on gender. There were no differences observed in saxagliptin pharmacokinetics between males and females. Compared to males, females had approximately 25% higher exposure values for the active metabolite than males, but this difference is unlikely to be of clinical relevance. Gender was not identified as a significant covariate on the apparent clearance of saxagliptin and its active metabolite in the population pharmacokinetic analysis.
Metformin hydrochloride:
Metformin pharmacokinetic parameters did not differ significantly between healthy subjects and patients with type 2 diabetes when analyzed according to gender (males=19, females=16). Similarly, in controlled clinical studies in patients with type 2 diabetes, the antihyperglycemic effect of metformin was comparable in males and females.
Geriatric
Saxagliptin:
No dosage adjustment is recommended based on age alone. Elderly subjects (65-80 years) had 23% and 59% higher geometric mean Cmax and geometric mean AUC values, respectively, for saxagliptin than young subjects (18-40 years). Differences in active metabolite pharmacokinetics between elderly and young subjects generally reflected the differences observed in saxagliptin pharmacokinetics. The difference between the pharmacokinetics of saxagliptin and the active metabolite in young and elderly subjects is likely due to multiple factors including declining renal function and metabolic capacity with increasing age. Age was not identified as a significant covariate on the apparent clearance of saxagliptin and its active metabolite in the population pharmacokinetic analysis.
Metformin hydrochloride:
Limited data from controlled pharmacokinetic studies of metformin in healthy elderly subjects suggest that total plasma clearance of metformin is decreased, the half-life is prolonged, and Cmax is increased, compared to healthy young subjects. From these data, it appears that the change in metformin pharmacokinetics with aging is primarily accounted for by a change in renal function.
Race and Ethnicity
Saxagliptin:
No dosage adjustment is recommended based on race. The population pharmacokinetic analysis compared the pharmacokinetics of saxagliptin and its active metabolite in 309 Caucasian subjects with 105 non-Caucasian subjects (consisting of six racial groups). No significant difference in the pharmacokinetics of saxagliptin and its active metabolite were detected between these two populations.
Metformin hydrochloride:
No studies of metformin pharmacokinetic parameters according to race have been performed. In controlled clinical studies of metformin in patients with type 2 diabetes, the antihyperglycemic effect was comparable in Whites (n=249), Blacks (n=51), and Hispanics (n=24).
Drug Interaction Studies
Specific pharmacokinetic drug interaction studies with KOMBIGLYZE XR have not been performed, although such studies have been conducted with the individual saxagliptin and metformin components.
In Vitro Assessment of Drug Interactions
In in vitro studies, saxagliptin and its active metabolite did not inhibit CYP1A2, 2A6, 2B6, 2C9, 2C19, 2D6, 2E1, or 3A4, or induce CYP1A2, 2B6, 2C9, or 3A4. Therefore, saxagliptin is not expected to alter the metabolic clearance of coadministered drugs that are metabolized by these enzymes. Saxagliptin is a P-glycoprotein (P-gp) substrate, but is not a significant inhibitor or inducer of P-gp.
In Vivo Assessment of Drug Interactions
Table 3. Effect of Coadministered Drug on Systemic Exposures of Saxagliptin and its Active Metabolite, 5-hydroxy Saxagliptin:
| Coadministered Drug | Dosage of Coadministered Drug* | Dosage of Saxagliptin* | Geometric Mean Ratio (ratio with/without coadministered drug) No Effect = 1.00 | ||
|---|---|---|---|---|---|
| AUC† | Cmax | ||||
| No dosing adjustments required for the following: | |||||
| Metformin | 1000 mg | 100 mg | saxagliptin 5-hydroxy saxagliptin | 0.98 0.99 | 0.79 0.88 |
| Glyburide | 5 mg | 10 mg | saxagliptin 5-hydroxy saxagliptin | 0.98 ND | 1.08 ND |
| Pioglitazone‡ | 45 mg QD for 10 days | 10 mg QD for 5 days | saxagliptin 5-hydroxy saxagliptin | 1.11 ND | 1.11 ND |
| Digoxin | 0.25 mg q6h first day followed by q12h second day followed by QD for 5 days | 10 mg QD for 7 days | saxagliptin 5-hydroxy saxagliptin | 1.05 1.06 | 0.99 1.02 |
| Dapagliflozin | 10 mg single dose | 5 mg single dose | saxagliptin 5-hydroxy saxagliptin | ↓1%↑9% | ↓7%↑6% |
| Simvastatin | 40 mg QD for 8 days | 10 mg QD for 4 days | saxagliptin 5-hydroxy saxagliptin | 1.12 1.02 | 1.21 1.08 |
| Diltiazem | 360 mg LA QD for 9 days | 10 mg | saxagliptin 5-hydroxy saxagliptin | 2.09 0.66 | 1.63 0.57 |
| Rifampin§ | 600 mg QD for 6 days | 5 mg | saxagliptin 5-hydroxy saxagliptin | 0.24 1.03 | 0.47 1.39 |
| Omeprazole | 40 mg QD for 5 days | 10 mg | saxagliptin 5-hydroxy saxagliptin | 1.13 ND | 0.98 ND |
| Aluminum hydroxide + magnesium hydroxide + simethicone | aluminum hydroxide: 2400 mg magnesium hydroxide: 2400 mg simethicone: 240 mg | 10 mg | saxagliptin 5-hydroxy saxagliptin | 0.97 ND | 0.74 ND |
| Famotidine | 40 mg | 10 mg | saxagliptin 5-hydroxy saxagliptin | 1.03 ND | 1.14 ND |
| Limit KOMBIGLYZE XR dose to 2.5 mg/1000 mg once daily when coadministered with strong CYP3A4/5 inhibitors [see Drug Interactions (7.1) and Dosage and Administration (2.2)]: | |||||
| Ketoconazole | 200 mg BID for 9 days | 100 mg | saxagliptin 5-hydroxy saxagliptin | 2.45 0.12 | 1.62 0.05 |
| Ketoconazole | 200 mg BID for 7 days | 20 mg | saxagliptin 5-hydroxy saxagliptin | 3.67 ND | 2.44 ND |
* Single dose unless otherwise noted. The 10 mg saxagliptin dose is not an approved dosage.
† AUC = AUC for drugs given as single dose and AUC = AUC for drugs given in multiple doses.
‡ Results exclude one subject.
§ The plasma dipeptidyl peptidase-4 (DPP4) activity inhibition over a 24-hour dose interval was not affected by rifampin.
Table 4. Effect of Saxagliptin on Systemic Exposures of Coadministered Drugs:
| Coadministered Drug | Dosage of Coadministered Drug* | Dosage of Saxagliptin* | Geometric Mean Ratio (ratio with/without saxagliptin) No Effect = 1.00 | ||
|---|---|---|---|---|---|
| AUC† | Cmax | ||||
| No dosing adjustments required for the following: | |||||
| Metformin | 1000 mg | 100 mg | metformin | 1.20 | 1.09 |
| Glyburide | 5 mg | 10 mg | glyburide | 1.06 | 1.16 |
| Pioglitazone‡ | 45 mg QD for 10 days | 10 mg QD for 5 days | pioglitazone hydroxy- pioglitazone | 1.08 ND | 1.14 ND |
| Digoxin | 0.25 mg q6h first day followed by q12h second day followed by QD for 5 days | 10 mg QD for 7 days | digoxin | 1.06 | 1.09 |
| Simvastatin | 40 mg QD for 8 days | 10 mg QD for 4 days | simvastatin simvastatin acid | 1.04 1.16 | 0.88 1.00 |
| Diltiazem | 360 mg LA QD for 9 days | 10 mg | diltiazem | 1.10 | 1.16 |
| Ketoconazole | 200 mg BID for 9 days | 100 mg | ketoconazole | 0.87 | 0.84 |
| Ethinyl estradiol and norgestimate | ethinyl estradiol 0.035 mg and norgestimate 0.250 mg for 21 days | 5 mg QD for 21 days | ethinyl estradiol norelgestromin norgestrel | 1.07 1.10 1.13 | 0.98 1.09 1.17 |
* Single dose unless otherwise noted. The 10 mg saxagliptin dose is not an approved dosage.
† AUC = AUC for drugs given as single dose and AUC = AUC for drugs given in multiple doses.
‡ Results include all subjects.
Table 5. Effect of Coadministered Drug on Plasma Metformin Systemic Exposure:
| Coadministered Drug | Dose of Coadministered Drug* | Dose of Metformin* | Geometric Mean Ratio (ratio with/without coadministered drug) No Effect = 1.00 | ||
|---|---|---|---|---|---|
| AUC† | Cmax | ||||
| No dosing adjustments required for the following: | |||||
| Glyburide | 5 mg | 850 mg | metformin | 0.91‡ | 0.93‡ |
| Furosemide | 40 mg | 850 mg | metformin | 1.09‡ | 1.22‡ |
| Nifedipine | 10 mg | 850 mg | metformin | 1.16 | 1.21 |
| Propranolol | 40 mg | 850 mg | metformin | 0.90 | 0.94 |
| Ibuprofen | 400 mg | 850 mg | metformin | 1.05‡ | 1.07‡ |
| Drugs that are eliminated by renal tubular secretion may increase the accumulation of metformin [see Drug Interactions (7.3)]. | |||||
| Cimetidine | 400 mg | 850 mg | metformin | 1.40 | 1.61 |
* All metformin and coadministered drugs were given as single doses.
† AUC = AUC
‡ Ratio of arithmetic means
Table 6. Effect of Metformin on Coadministered Drug Systemic Exposure:
| Coadministered Drug | Dose of Coadministered Drug* | Dose of Metformin* | Geometric Mean Ratio (ratio with/without metformin) No Effect = 1.00 | ||
|---|---|---|---|---|---|
| AUC† | Cmax | ||||
| No dosing adjustments required for the following: | |||||
| Glyburide | 5 mg | 850 mg | glyburide | 0.78‡ | 0.63‡ |
| Furosemide | 40 mg | 850 mg | furosemide | 0.87‡ | 0.69‡ |
| Nifedipine | 10 mg | 850 mg | nifedipine | 1.10§ | 1.08 |
| Propranolol | 40 mg | 850 mg | propranolol | 1.01§ | 1.02 |
| Ibuprofen | 400 mg | 850 mg | ibuprofen | 0.97¶ | 1.01¶ |
| Cimetidine | 400 mg | 850 mg | cimetidine | 0.95§ | 1.01 |
* All metformin and coadministered drugs were given as single doses.
† AUC = AUC unless otherwise noted.
‡ Ratio of arithmetic means, p-value of difference <0.05.
§ AUC reported.
¶ Ratio of arithmetic means.
13.1. Carcinogenesis, Mutagenesis, Impairment of Fertility
KOMBIGLYZE XR
No animal studies have been conducted with the combined products in KOMBIGLYZE XR to evaluate carcinogenesis, mutagenesis, or impairment of fertility. The following data are based on studies with saxagliptin and metformin administered individually.
Saxagliptin
Carcinogenesis
Carcinogenicity was evaluated in 2-year studies conducted in CD-1 mice and Sprague-Dawley rats. Saxagliptin did not increase the incidence of tumors in mice dosed orally at 50, 250, and 600 mg/kg up to 870-times (males) and 1165-times (females) the 5 mg/day clinical dose, based on AUC. Saxagliptin did not increase the incidence of tumors in rats dosed orally at 25, 75, 150, and 300 mg/kg up to 355-times (males) and 2217-times (females) the 5 mg/day clinical dose, based on AUC.
Mutagenesis
Saxagliptin was not mutagenic or clastogenic in a battery of genotoxicity tests (Ames bacterial mutagenesis, human and rat lymphocyte cytogenetics, rat bone marrow micronucleus and DNA repair assays). The active metabolite of saxagliptin was not mutagenic in an Ames bacterial assay.
Impairment of Fertility
Saxagliptin administered to rats had no effect on fertility or the ability to maintain a litter at exposures up to 603-times and 776-times the 5 mg clinical dose in males and females, based on AUC.
Metformin hydrochloride
Carcinogenesis
Long-term carcinogenicity studies have been performed in rats (dosing duration of 104 weeks) and mice (dosing duration of 91 weeks) at doses up to and including 900 mg/kg/day and 1500 mg/kg/day, respectively. These doses are both approximately 4 times the maximum recommended human daily dose of 2000 mg based on body surface area comparisons. No evidence of carcinogenicity with metformin was found in either male or female mice. Similarly, there was no tumorigenic potential observed with metformin in male rats. There was, however, an increased incidence of benign stromal uterine polyps in female rats treated with 900 mg/kg/day.
Mutagenesis
There was no evidence of a mutagenic potential of metformin in the following in vitro tests: Ames test (S. typhimurium), gene mutation test (mouse lymphoma cells), or chromosomal aberrations test (human lymphocytes). Results in the in vivo mouse micronucleus test were also negative.
Impairment of Fertility
Fertility of male or female rats was unaffected by metformin when administered at doses as high as 600 mg/kg/day, which is approximately 3 times the maximum recommended human daily dose based on body surface area comparisons.
13.2. Animal Toxicology and/or Pharmacology
Saxagliptin
Saxagliptin produced adverse skin changes in the extremities of cynomolgus monkeys (scabs and/or ulceration of tail, digits, scrotum, and/or nose). Skin lesions were reversible within exposure approximately 20-times the 5 mg clinical dose, but in some cases were irreversible and necrotizing at higher exposures. Adverse skin changes were not observed at exposures similar to (1- to 3-times) the 5 mg clinical dose. Clinical correlates to skin lesions in monkeys have not been observed in human clinical trials of saxagliptin.
14. Clinical Studies
There have been no clinical efficacy or safety studies conducted with KOMBIGLYZE XR to characterize its effect on A1C reduction. Bioequivalence of KOMBIGLYZE XR with coadministered saxagliptin and metformin hydrochloride extended-release tablets has been demonstrated; however, relative bioavailability studies between KOMBIGLYZE XR and coadministered saxagliptin and metformin hydrochloride immediate-release tablets have not been conducted. The metformin hydrochloride extended-release tablets and metformin hydrochloride immediate-release tablets have a similar extent of absorption (as measured by AUC) while peak plasma levels of extended-release tablets are approximately 20% lower than those of immediate-release tablets at the same dose.
14.1 Glycemic Efficacy Trials
The coadministration of saxagliptin and metformin immediate-release tablets has been studied in adults with type 2 diabetes inadequately controlled on metformin alone and in treatment-naive patients inadequately controlled on diet and exercise alone. In these two trials, treatment with saxagliptin dosed in the morning plus metformin immediate-release tablets at all doses produced clinically relevant and statistically significant improvements in A1C, fasting plasma glucose (FPG), and 2-hour postprandial glucose (PPG) following a standard oral glucose tolerance test (OGTT), compared to control. Reductions in A1C were seen across subgroups including gender, age, race, and baseline BMI.
In these two trials, decrease in body weight in the treatment groups given saxagliptin in combination with metformin immediate-release was similar to that in the groups given metformin immediate-release alone. Saxagliptin plus metformin immediate-release was not associated with significant changes from baseline in fasting serum lipids compared to metformin alone.
The coadministration of saxagliptin and metformin immediate-release tablets has also been evaluated in an active-controlled trial comparing add-on therapy with saxagliptin to glipizide in 858 patients inadequately controlled on metformin alone, in a placebo-controlled trial where a subgroup of 314 patients inadequately controlled on insulin plus metformin received add-on therapy with saxagliptin or placebo, a trial comparing saxagliptin to placebo in 257 patients inadequately controlled on metformin plus a sulfonylurea, and a trial comparing saxagliptin to placebo in 315 patients inadequately controlled on dapagliflozin and metformin.
In a 24-week, double-blind, randomized trial, patients treated with metformin immediate-release 500 mg twice daily for at least 8 weeks were randomized to continued treatment with metformin immediate-release 500 mg twice daily or to metformin extended-release either 1000 mg once daily or 1500 mg once daily. The mean change in A1C from baseline to Week 24 was 0.1% (95% confidence interval 0%, 0.3%) for the metformin immediate-release treatment arm, 0.3% (95% confidence interval 0.1%, 0.4%) for the 1000 mg metformin extended-release treatment arm, and 0.1% (95% confidence interval 0%, 0.3%) for the 1500 mg metformin extended-release treatment arm. Results of this trial suggest that patients receiving metformin immediate-release treatment may be safely switched to metformin extended-release once daily at the same total daily dose, up to 2000 mg once daily. Following a switch from metformin immediate-release to metformin extended-release, glycemic control should be closely monitored and dosage adjustments made accordingly.
Saxagliptin Morning and Evening Dosing
A 24-week monotherapy trial was conducted to assess a range of dosing regimens for saxagliptin. Treatment-naive patients with inadequately controlled diabetes (A1C ≥7% to ≤10%) underwent a 2-week, single-blind diet, exercise, and placebo lead-in period. A total of 365 patients were randomized to 2.5 mg every morning, 5 mg every morning, 2.5 mg with possible titration to 5 mg every morning, or 5 mg every evening of saxagliptin, or placebo. Patients who failed to meet specific glycemic goals during the study were treated with metformin rescue therapy added on to placebo or saxagliptin; the number of patients randomized per treatment group ranged from 71 to 74.
Treatment with either saxagliptin 5 mg every morning or 5 mg every evening provided significant improvements in A1C versus placebo (mean placebo-corrected reductions of −0.4% and −0.3%, respectively).
Coadministration of Saxagliptin with Metformin Immediate-Release in Treatment-Naive Patients
A total of 1306 treatment-naive patients with type 2 diabetes mellitus participated in this 24-week, randomized, double-blind, active-controlled trial to evaluate the efficacy and safety of saxagliptin coadministered with metformin immediate-release in patients with inadequate glycemic control (A1C ≥8% to ≤12%) on diet and exercise alone. Patients were required to be treatment-naive to be enrolled in this study.
Patients who met eligibility criteria were enrolled in a single-blind, 1-week, dietary and exercise placebo lead-in period. Patients were randomized to one of four treatment arms: saxagliptin 5 mg + metformin immediate-release 500 mg, saxagliptin 10 mg + metformin immediate-release 500 mg, saxagliptin 10 mg + placebo, or metformin immediate-release 500 mg + placebo (the maximum recommended approved saxagliptin dose is 5 mg daily; the 10 mg daily dose of saxagliptin does not provide greater efficacy than the 5 mg daily dose and the 10 mg saxagliptin dosage is not an approved dosage). Saxagliptin was dosed once daily. In the 3 treatment groups using metformin immediate-release, the metformin dose was up-titrated weekly in 500 mg per day increments, as tolerated, to a maximum of 2000 mg per day based on FPG. Patients who failed to meet specific glycemic goals during this study were treated with pioglitazone rescue as add-on therapy.
Coadministration of saxagliptin 5 mg plus metformin immediate-release provided significant improvements in A1C, FPG, and PPG compared with placebo plus metformin immediate-release (Table 7).
Table 7. Glycemic Parameters at Week 24 in a Placebo-Controlled Trial of Saxagliptin Coadministration with Metformin Immediate-Release in Treatment-Naive Patients:*
| Efficacy Parameter | Saxagliptin 5 mg + Metformin N=320 | Placebo + Metformin N=328 |
|---|---|---|
| Hemoglobin A1C (%) | N=306 | N=313 |
| Baseline (mean) | 9.4 | 9.4 |
| Change from baseline (adjusted mean†) | −2.5 | −2.0 |
| Difference from placebo + metformin (adjusted mean†) | −0.5‡ | |
| 95% Confidence Interval | (−0.7, −0.4) | |
| Percent of patients achieving A1C <7% | 60%§ (185/307) | 41% (129/314) |
| Fasting Plasma Glucose (mg/dL) | N=315 | N=320 |
| Baseline (mean) | 199 | 199 |
| Change from baseline (adjusted mean†) | −60 | −47 |
| Difference from placebo + metformin (adjusted mean†^) | −13§ | |
| 95% Confidence Interval | (−19, −6) | |
| 2-hour Postprandial Glucose (mg/dL) | N=146 | N=141 |
| Baseline (mean) | 340 | 355 |
| Change from baseline (adjusted mean†) | −138 | −97 |
| Difference from placebo + metformin (adjusted mean†) | −41§ | |
| 95% Confidence Interval | (−57, −25) |
* Intent-to-treat population using last observation on study or last observation prior to pioglitazone rescue therapy for patients needing rescue.
† Least squares mean adjusted for baseline value.
‡ p-value <0.0001 compared to placebo + metformin.
§ p-value <0.05 compared to placebo + metformin.
Addition of Saxagliptin to Metformin Immediate-Release
A total of 743 patients with type 2 diabetes participated in this 24-week, randomized, double-blind, placebo-controlled trial to evaluate the efficacy and safety of saxagliptin in combination with metformin immediate-release in patients with inadequate glycemic control (A1C ≥7% and ≤10%) on metformin alone. To qualify for enrollment, patients were required to be on a stable dose of metformin (1500-2550 mg daily) for at least 8 weeks.
Patients who met eligibility criteria were enrolled in a single-blind, 2-week, dietary and exercise placebo lead-in period during which patients received metformin immediate-release at their pre-study dose, up to 2500 mg daily, for the duration of the study. Following the lead-in period, eligible patients were randomized to 2.5 mg, 5 mg, or 10 mg of saxagliptin or placebo in addition to their current dose of open-label metformin immediate-release (the maximum recommended approved saxagliptin dose is 5 mg daily; the 10 mg daily dose of saxagliptin does not provide greater efficacy than the 5 mg daily dose and the 10 mg dosage is not an approved dosage). Patients who failed to meet specific glycemic goals during the study were treated with pioglitazone rescue therapy, added on to existing study medications. Dose titrations of saxagliptin and metformin immediate-release were not permitted.
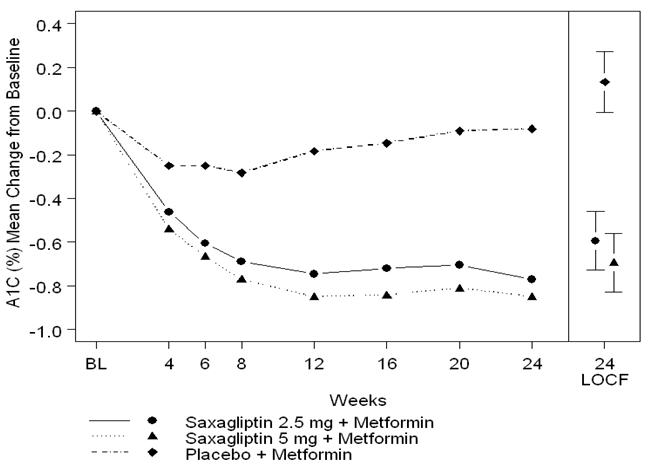
Saxagliptin 2.5 mg and 5 mg add-on to metformin immediate-release provided significant improvements in A1C, FPG, and PPG compared with placebo add-on to metformin immediate-release (Table 8). Mean changes from baseline for A1C over time and at endpoint are shown in Figure 1. The proportion of patients who discontinued for lack of glycemic control or who were rescued for meeting prespecified glycemic criteria was 15% in the saxagliptin 2.5 mg add-on to metformin immediate-release group, 13% in the saxagliptin 5 mg add-on to metformin immediate-release group, and 27% in the placebo add-on to metformin immediate-release group.
Table 8. Glycemic Parameters at Week 24 in a Placebo-Controlled Study of Saxagliptin as Add-On Combination Therapy with Metformin Immediate-Release*:
| Efficacy Parameter | Saxagliptin 2.5 mg + Metformin N=192 | Saxagliptin 5 mg + Metformin N=191 | Placebo + Metformin N=179 |
|---|---|---|---|
| Hemoglobin A1C (%) | N=186 | N=186 | N=175 |
| Baseline (mean) | 8.1 | 8.1 | 8.1 |
| Change from baseline (adjusted mean†) | −0.6 | −0.7 | +0.1 |
| Difference from placebo (adjusted mean†) | −0.7‡ | −0.8‡ | |
| 95% Confidence Interval | (−0.9, −0.5) | (−1.0, −0.6) | |
| Percent of patients achieving A1C <7% | 37%§ (69/186) | 44%§ (81/186) | 17% (29/175) |
| Fasting Plasma Glucose (mg/dL) | N=188 | N=187 | N=176 |
| Baseline (mean) | 174 | 179 | 175 |
| Change from baseline (adjusted mean†) | −14 | −22 | +1 |
| Difference from placebo (adjusted mean†) | −16§ | −23§ | |
| 95% Confidence Interval | (−23, −9) | (−30, −16) | |
| 2-hour Postprandial Glucose (mg/dL) | N=155 | N=155 | N=135 |
| Baseline (mean) | 294 | 296 | 295 |
| Change from baseline (adjusted mean†) | −62 | −58 | −18 |
| Difference from placebo (adjusted mean†) | −44§ | −40§ | |
| 95% Confidence Interval | (−60, −27) | (−56, −24) |
* Intent-to-treat population using last observation on study or last observation prior to pioglitazone rescue therapy for patients needing rescue.
† Least squares mean adjusted for baseline value.
‡ p-value <0.0001 compared to placebo + metformin.
§ p-value <0.05 compared to placebo + metformin.
Figure 1. Mean Change from Baseline in A1C in a Placebo-Controlled Trial of Saxagliptin as Add-On Combination Therapy with Metformin Immediate-Release*:
* Includes patients with a baseline and week 24 value.
Week 24 (LOCF) includes intent-to-treat population using last observation on study prior to pioglitazone rescue therapy for patients needing rescue. Mean change from baseline is adjusted for baseline value.
Saxagliptin Add-On Combination Therapy with Metformin Immediate-Release versus Glipizide Add-On Combination Therapy with Metformin Immediate-Release
In this 52-week, active-controlled trial, a total of 858 patients with type 2 diabetes and inadequate glycemic control (A1C >6.5% and ≤10%) on metformin immediate-release alone were randomized to double-blind add-on therapy with saxagliptin or glipizide. Patients were required to be on a stable dose of metformin immediate-release (at least 1500 mg daily) for at least 8 weeks prior to enrollment.
Patients who met eligibility criteria were enrolled in a single-blind, 2-week, dietary and exercise placebo lead-in period during which patients received metformin immediate-release (1500-3000 mg based on their pre-study dose). Following the lead-in period, eligible patients were randomized to 5 mg of saxagliptin or 5 mg of glipizide in addition to their current dose of open-label metformin immediate-release. Patients in the glipizide plus metformin immediate-release group underwent blinded titration of the glipizide dose during the first 18 weeks of the trial up to a maximum glipizide dose of 20 mg per day. Titration was based on a goal FPG ≤110 mg/dL or the highest tolerable glipizide dose. Fifty percent (50%) of the glipizide-treated patients were titrated to the 20-mg daily dose; 21% of the glipizide-treated patients had a final daily glipizide dose of 5 mg or less. The mean final daily dose of glipizide was 15 mg.
After 52 weeks of treatment, saxagliptin and glipizide resulted in similar mean reductions from baseline in A1C when added to metformin immediate-release therapy (Table 9). This conclusion may be limited to patients with baseline A1C comparable to those in the trial (91% of patients had baseline A1C <9%).
From a baseline mean body weight of 89 kg, there was a statistically significant mean reduction of 1.1 kg in patients treated with saxagliptin compared to a mean weight gain of 1.1 kg in patients treated with glipizide (p<0.0001).
Table 9. Glycemic Parameters at Week 52 in an Active-Controlled Trial of Saxagliptin versus Glipizide in Combination with Metformin Immediate-Release*:
| Efficacy Parameter | Saxagliptin 5 mg + Metformin N=428 | Titrated Glipizide + Metformin N=430 |
|---|---|---|
| Hemoglobin A1C (%) | N=423 | N=423 |
| Baseline (mean) | 7.7 | 7.6 |
| Change from baseline (adjusted mean†) | −0.6 | −0.7 |
| Difference from glipizide + metformin (adjusted mean†) | 0.1 | |
| 95% Confidence Interval | (−0.02, 0.2)‡ | |
| Fasting Plasma Glucose (mg/dL) | N=420 | N=420 |
| Baseline (mean) | 162 | 161 |
| Change from baseline (adjusted mean†) | −9 | −16 |
| Difference from glipizide + metformin (adjusted mean†) | 6 | |
| 95% Confidence Interval | (2, 11)§ |
‡ Saxagliptin + metformin is considered non-inferior to glipizide + metformin because the upper limit of this confidence interval is less than the prespecified non-inferiority margin of 0.35%.
§ Significance not tested.
Saxagliptin Add-On Combination Therapy with Insulin (with or without Metformin Immediate-Release)
A total of 455 patients with type 2 diabetes participated in this 24-week, randomized, double-blind, placebo-controlled trial to evaluate the efficacy and safety of saxagliptin in combination with insulin in patients with inadequate glycemic control (A1C ≥7.5% and ≤11%) on insulin alone (N=141) or on insulin in combination with a stable dose of metformin immediate-release (N=314). Patients were required to be on a stable dose of insulin (≥30 units to ≤150 units daily) with ≤20% variation in total daily dose for ≥8 weeks prior to screening. Patients entered the trial on intermediate- or long-acting (basal) insulin or premixed insulin. Patients using short-acting insulins were excluded unless the short-acting insulin was administered as part of a premixed insulin.
Patients who met eligibility criteria were enrolled in a single-blind, four-week, dietary and exercise placebo lead-in period during which patients received insulin (and metformin immediate-release if applicable) at their pretrial dose(s). Following the lead-in period, eligible patients were randomized to add-on therapy with either saxagliptin 5 mg or placebo. Doses of the antidiabetic therapies were to remain stable but patients were rescued and allowed to adjust the insulin regimen if specific glycemic goals were not met or if the investigator learned that the patient had self-increased the insulin dose by >20%. Data after rescue were excluded from the primary efficacy analyses.
Add-on therapy with saxagliptin 5 mg provided significant improvements from baseline to Week 24 in A1C and PPG compared with add-on placebo (Table 10). Similar mean reductions in A1C versus placebo were observed for patients using saxagliptin 5 mg add-on to insulin alone and saxagliptin 5 mg add-on to insulin in combination with metformin immediate-release (−0.4% and −0.4%, respectively). The percentage of patients who discontinued for lack of glycemic control or who were rescued was 23% in the saxagliptin group and 32% in the placebo group.
The mean daily insulin dose at baseline was 53 units in patients treated with saxagliptin 5 mg and 55 units in patients treated with placebo. The mean change from baseline in daily dose of insulin was 2 units for the saxagliptin 5 mg group and 5 units for the placebo group.
Table 10. Glycemic Parameters at Week 24 in a Placebo-Controlled Trial of Saxagliptin as Add-On Combination Therapy with Insulin*:
| Efficacy Parameter | Saxagliptin 5 mg + Insulin (+/− Metformin) N=304 | Placebo Insulin (/− Metformin) N=151 |
|---|---|---|
| Hemoglobin A1C (%) | N=300 | N=149 |
| Baseline (mean) | 8.7 | 8.7 |
| Change from baseline (adjusted mean†) | −0.7 | −0.3 |
| Difference from placebo (adjusted mean†) | −0.4‡ | |
| 95% Confidence Interval | (−0.6, −0.2) | |
| 2-hour Postprandial Glucose (mg/dL) | N=262 | N=129 |
| Baseline (mean) | 251 | 255 |
| Change from baseline (adjusted mean†) | −27 | −4 |
| Difference from placebo (adjusted mean†) | −23§ | |
| 95% Confidence Interval | (−37, −9) |
* Intent-to-treat population using last observation on study or last observation prior to insulin rescue therapy for patients needing rescue.
† Least squares mean adjusted for baseline value and metformin use at baseline.
‡ p-value <0.0001 compared to placebo + insulin.
§ p-value <0.05 compared to placebo + insulin.
The change in fasting plasma glucose from baseline to Week 24 was also tested, but was not statistically significant. The percent of patients achieving an A1C <7% was 17% (52/300) with saxagliptin in combination with insulin compared to 7% (10/149) with placebo. Significance was not tested.
Saxagliptin Add-On Combination Therapy with Metformin plus Sulfonylurea
A total of 257 subjects with type 2 diabetes participated in this 24-week, randomized, double-blind, placebo-controlled trial to evaluate the efficacy and safety of saxagliptin in combination with metformin plus a sulfonylurea in patients with inadequate glycemic control (A1C ≥7% and ≤10%). Patients were to be on a stable combined dose of metformin extended-release or immediate-release (at maximum tolerated dose, with minimum dose for enrollment being 1500 mg) and a sulfonylurea (at maximum tolerated dose, with minimum dose for enrollment being ≥50% of the maximum recommended dose) for ≥8 weeks prior to enrollment.
Patients who met eligibility criteria were entered in a 2-week enrollment period to allow assessment of inclusion/exclusion criteria. Following the 2-week enrollment period, eligible patients were randomized to either double-blind saxagliptin (5 mg once daily) or double-blind matching placebo for 24 weeks. During the 24-week double-blind treatment period, patients were to receive metformin and a sulfonylurea at the same constant dose ascertained during enrollment. Sulfonylurea dose could be down titrated once in the case of a major hypoglycemic event or recurring minor hypoglycemic events. In the absence of hypoglycemia, titration (up or down) of study medication during the treatment period was prohibited.
Saxagliptin in combination with metformin plus a sulfonylurea provided significant improvements in A1C and PPG compared with placebo in combination with metformin plus a sulfonylurea (Table 11). The percentage of patients who discontinued for lack of glycemic control was 6% in the saxagliptin group and 5% in the placebo group.
Table 11. Glycemic Parameters at Week 24 in a Placebo-Controlled Trial of Saxagliptin as Add-On Combination Therapy with Metformin plus Sulfonylurea*:
| Efficacy Parameter | Saxagliptin 5 mg + Metformin plus Sulfonylurea N=129 | Placebo + Metformin plus Sulfonylurea N=128 |
|---|---|---|
| Hemoglobin A1C (%) | N=127 | N=127 |
| Baseline (mean) | 8.4 | 8.2 |
| Change from baseline (adjusted mean†) | −0.7 | −0.1 |
| Difference from placebo (adjusted mean†) | −0.7‡ | |
| 95% Confidence Interval | (−0.9, −0.5) | |
| 2-hour Postprandial Glucose (mg/dL) | N=115 | N=113 |
| Baseline (mean) | 268 | 262 |
| Change from baseline (adjusted mean†) | −12 | 5 |
| Difference from placebo (adjusted mean†) | −17§ | |
| 95% Confidence Interval | (−32, −2) |
* Intent-to-treat population using last observation prior to discontinuation.
† Least squares mean adjusted for baseline value.
‡ p-value <0.0001 compared to placebo + metformin plus sulfonylurea.
§ p-value <0.05 compared to placebo + metformin plus sulfonylurea.
The change in fasting plasma glucose from baseline to Week 24 was also tested, but was not statistically significant. The percent of patients achieving an A1C <7% was 31% (39/127) with saxagliptin in combination with metformin plus a sulfonylurea compared to 9% (12/127) with placebo. Significance was not tested.
Saxagliptin Add-on Combination Therapy with Metformin plus an SGLT2 Inhibitor
A total of 315 patients with type 2 diabetes participated in this 24-week randomized, double-blind, placebo-controlled trial to evaluate the efficacy and safety of saxagliptin added to dapagliflozin (an SGLT2 inhibitor) and metformin in patients with a baseline of HbA1c ≥7% to ≤10.5%. The mean age of these subjects was 54.6 years, 1.6% were 75 years or older and 52.7% were female. The population was 87.9% White, 6.3% Black or African American, 4.1% Asian, and 1.6% Other race. At baseline the population had diabetes for an average of 7.7 years and a mean HbA1c of 7.9%. The mean eGFR at baseline was 93.4 mL/min/1.73 m². Patients were required to be on a stable dose of metformin (≥1500 mg per day) for at least 8 weeks prior to enrollment. Eligible subjects who completed the screening period entered the lead in treatment period, which included 16 weeks of open-label metformin and 10 mg dapagliflozin treatment. Following the lead-in period, eligible patients were randomized to saxagliptin 5 mg (N=153) or placebo (N=162).
The group treated with add-on saxagliptin had statistically significant greater reductions in HbA1c from baseline versus the group treated with placebo (see Table 12).
Table 12. HbA1c Change from Baseline at Week 24 in a Placebo-Controlled Trial of Saxagliptin as Add-On to Dapagliflozin and Metformin*:
| Saxagliptin 5 mg (N=153)† | Placebo (N=162)† | |
|---|---|---|
| In combination with Dapagliflozin and Metformin | ||
| Hemoglobin A1C (%)‡ | ||
| Baseline (mean) | 8.0 | 7.9 |
| Change from baseline (adjusted mean§)• 95% Confidence Interval | −0.5 (−0.6, −0.4) | −0.2 (−0.3, −0.1) |
| Difference from placebo (adjusted mean)• 95% Confidence Interval | −0.4¶ (−0.5, −0.2) | |
* There were 6.5% (n=10) of randomized subjects in the saxagliptin arm and 3.1% (n=5) in the placebo arm for whom change from baseline HbA1c data was missing at week 24. Of the subjects who discontinued study medication early, 9.1% (1 of 11) in the saxagliptin arm and 16.7% (1 of 6) in the placebo arm had HbA1c measured at week 24.
† Number of randomized and treated patients.
‡ Analysis of Covariance including all post-baseline data regardless of rescue or treatment discontinuation. Model estimates calculated using multiple imputation to model washout of the treatment effect using placebo data for all subjects having missing week 24 data.
§ Least squares mean adjusted for baseline value.
¶ p-value <0.0001
The known proportion of patients achieving HbA1c <7% at Week 24 was 35.3% in the saxagliptin-treated group compared to 23.1% in the placebo-treated group.
14.2 Cardiovascular Safety Trial
The cardiovascular risk of saxagliptin was evaluated in SAVOR, a multicenter, multinational, randomized, double-blind study comparing saxagliptin (N=8280) to placebo (N=8212), both administered in combination with standard of care, in adult patients with type 2 diabetes at high risk for atherosclerotic cardiovascular disease. Of the randomized study subjects, 97.5% completed the trial, and the median duration of follow-up was approximately 2 years. The trial was event-driven, and patients were followed until a sufficient number of events were accrued.
Subjects were at least 40 years of age, had A1C ≥6.5%, and multiple risk factors (21% of randomized subjects) for cardiovascular disease (age ≥55 years for men and ≥60 years for women plus at least one additional risk factor of dyslipidemia, hypertension, or current cigarette smoking) or established (79% of the randomized subjects) cardiovascular disease defined as a history of ischemic heart disease, peripheral vascular disease, or ischemic stroke. The majority of subjects were male (67%) and Caucasian (75%) with a mean age of 65 years. Approximately 16% of the population had moderate (estimated glomerular filtration rate [eGFR] ≥30 to ≤50 mL/min) to severe (eGFR <30 mL/min) renal impairment, and 13% had a prior history of heart failure. Subjects had a median duration of type 2 diabetes mellitus of approximately 10 years, and a mean baseline A1C level of 8.0%. Approximately 5% of subjects were treated with diet and exercise only at baseline. Overall, the use of diabetes medications was balanced across treatment groups (metformin 69%, insulin 41%, sulfonylureas 40%, and TZDs 6%). The use of cardiovascular disease medications was also balanced (angiotensin-converting enzyme [ACE] inhibitors or angiotensin receptor blockers [ARBs] 79%, statins 78%, aspirin 75%, beta-blockers 62%, and non-aspirin antiplatelet medications 24%).
The primary analysis in SAVOR was time to first occurrence of a Major Adverse Cardiac Event (MACE). A major adverse cardiac event in SAVOR was defined as a cardiovascular death, or a nonfatal myocardial infarction (MI) or a nonfatal ischemic stroke. The study was designed as a non-inferiority trial with a pre-specified risk margin of 1.3 for the hazard ratio of MACE, and was also powered for a superiority comparison if non-inferiority was demonstrated.
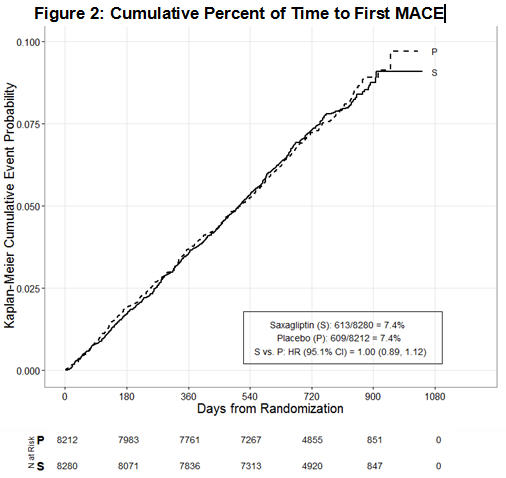
The results of SAVOR, including the contribution of each component to the primary composite endpoint are shown in Table 13. The incidence rate of MACE was similar in both treatment arms: 3.8 MACE per 100 patient-years on placebo vs. 3.8 MACE per 100 patient-years on saxagliptin. The estimated hazard ratio of MACE associated with saxagliptin relative to placebo was 1.00 with a 95.1% confidence interval of (0.89, 1.12). The upper bound of this confidence interval, 1.12, excluded a risk margin larger than 1.3.
Table 13. Major Adverse Cardiovascular Events (MACE) by Treatment Group in the SAVOR Trial:
| Saxagliptin | Placebo | Hazard Ratio | |||
|---|---|---|---|---|---|
| Number of ubjects (%) | Rate per 00 PY | Number of ubjects (%) | Rate per 00 PY | (95.1% CI) | |
| Composite of first event of CV death, non-fatal MI or non-fatal ischemic stroke (MACE) | N=8280 | Total PY = 16308.8 | N=8212 | Total PY = 16156.0 | |
| 613 (7.4) | 3.8 | 609 (7.4) | 3.8 | 1.00 (0.89, 1.12) | |
| CV death | 245 (3.0) | 1.5 | 234 (2.8) | 1.4 | |
| Non-fatal MI | 233 (2.8) | 1.4 | 260 (3.2) | 1.6 | |
| Non-fatal ischemic stroke | 135 (1.6) | 0.8 | 115 (1.4) | 0.7 | |
The Kaplan-Meier-based cumulative event probability is presented in Figure 2 for time to first occurrence of the primary MACE composite endpoint by treatment arm. The curves for both saxagliptin and placebo arms are close together throughout the duration of the trial. The estimated cumulative event probability is approximately linear for both arms, indicating that the incidence of MACE for both arms was constant over the trial duration.
Figure 2. Cumulative Percent of Time to First MACE:
Vital status was obtained for 99% of subjects in the trial. There were 798 deaths in the SAVOR trial. Numerically more patients (5.1%) died in the saxagliptin group than in the placebo group (4.6%). The risk of deaths from all cause (Table 14) was not statistically different between the treatment groups (HR: 1.11; 95.1% CI: 0.96, 1.27).
Table 14. All-Cause Mortality by Treatment Group in the SAVOR Study:
| Saxagliptin | Placebo | Hazard Ratio | |||
|---|---|---|---|---|---|
| Number of Subjects (%) | Rate per 100 PY | Number of Subjects (%) | Rate per 100 PY | (95.1% CI) | |
| N=8280 | PY=16645.3 | N=8212 | PY=16531.5 | ||
| All-cause mortality | 420 (5.1) | 2.5 | 378 (4.6) | 2.3 | 1.11 (0.96, 1.27) |
| CV death | 269 (3.2) | 1.6 | 260 (3.2) | 1.6 | |
| Non-CV death | 151 (1.8) | 0.9 | 118 (1.4) | 0.7 | |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.